White Matter Disturbances in Bipolar Disorder
At the 2020 meeting of the International Society for Bipolar Disorders, researcher Clare Beasley described the cellular and molecular underpinnings of the white matter abnormalities typically seen in children and adults with bipolar disorder. Researchers consistently see white matter abnormalities in neuroimaging studies of bipolar disorder, but not much is understood about what creates these deficits.
Beasley and colleagues studied autopsy specimens and found that compared to controls, people with bipolar disorder had a number of abnormalities affecting glial cells, lipid composition, and axons.
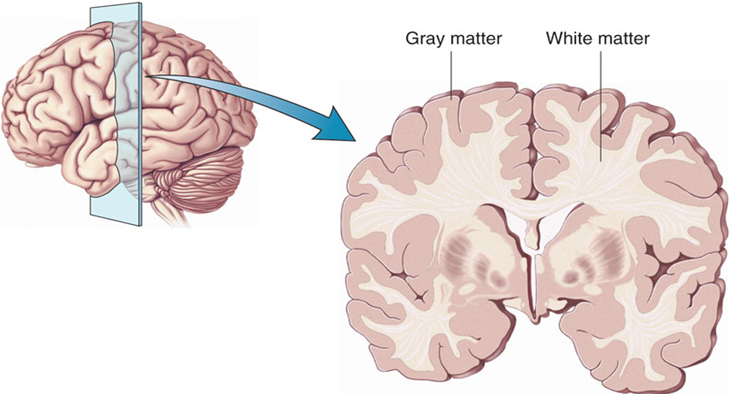
The researchers found increased density of oligodendrocytes (glial cells that produce the myelin that wraps around axons, the long fibers of nerve cells where impulses travel out to other cells) and an associated protein called CNP in the prefrontal cortex. The myelin is what makes up white matter, while gray matter consists of cell bodies of neurons and glial cells.
People with bipolar disorder also had differently-shaped astrocytes, another type of glial cell that abuts synapses. The researchers found changes in lipid composition, including phospholipid and fatty acid levels, in the white matter of people with bipolar disorder. There were also problems with axons. Beasley and colleagues noted lower density of axon-associated proteins, which are involved in transport of substances along the axons in people with bipolar disorder.
The authors conclude that these data implicate specific disturbances in oligodendrocytes and axonal function associated with the white matter alterations usually seen in neuroimages of people with bipolar disorder.
Endocannabinoid System May Help Explain Tourette Syndrome

Endocannabinoids are neurotransmitters produced by the human body that attach to cannabinoid receptors in the brain, the same receptors that are affected by the consumption of cannabis products.
Tourette syndrome, a neurodevelopmental disorder characterized by tics and psychological symptoms, is probably caused by some dysfunction involving the neurotransmitter dopamine. The syndrome is usually treated with dopamine receptor blockers but is also eased by cannabis use and treatment with THC, the main psychoactive component in cannabis. Recently, researchers set out to determine whether concentrations of endocannabinoids in the cerebrospinal system are related to Tourette syndrome.
In an article published in the journal Neuropsychopharmacology in 2020, researcher Kirsten R. Müller-Vahl and colleagues report that endocannabinoid concentrations were significantly higher in the cerebrospinal fluid of 20 people with Tourette’s syndrome than in 19 control participants without Tourette’s.
The researchers found elevations in the endocannabinoids AEA and 2-AG, the endocannabinoid-like ligand PEA, and the metabolite AA in the participants with Tourette’s syndrome. Levels of 2-AG in the cerebrospinal fluid correlated with severity of attention-deficit hyperactivity disorder symptoms, an aspect of the syndrome.
It is possible that higher concentrations of endocannabinoids are present in the syndrome because they compensate for the overactive influence of dopamine. This explanation would fit with the effectiveness of cannabis in treating Tourette’s. However, that has not yet been determined, and it is also possible that the endocannabinoids are a reaction to dysfunction involving other neurotransmitters, are incidental to the syndrome, or in the best case that they are a direct cause of the syndrome.
Müller-Vahl and colleagues suggest that based on the effectiveness of cannabis in treating Tourette’s, it may turn out that the syndrome is a sort of endocannabinoid deficiency. They believe this hypothesis is not counteracted by the high levels of cannabinoids they found in Tourette’s patients in this study, because these high levels may be accompanied by a reduced number or reduced sensitivity of the cannabinoid receptors or overactivity in the enzymes that break down endocannabinoids, such that it is difficult to maintain normal levels of these neurotransmitters.
Translocator Protein Levels in Brain Predict Response to Anti-Inflammatory Celecoxib in Major Depressive Disorder
Gliosis describes changes in glia that result from damage to the central nervous system. Researchers can use PET scans (positron emission tomography) to measure the extent of gliosis in the brain. But a new study explored whether these PET scans could instead be used to determine who might respond to a given medication.
Researcher Sophia Attwells and colleagues reported in the journal Biological Psychiatry in 2020 that people with high levels of translocator protein (TSPO), a measure of gliosis and inflammation, had a better antidepressant response to the anti-inflammatory drug celecoxib than patients who started out with lower levels of TSPO.
The study participants, who had treatment-resistant depression, all received 200mg of the anti-inflammatory drug celecoxib twice/day for eight weeks on an open (non-blind) basis. Before they began taking celecoxib, the participants received PET scans to measure translocator protein total distribution volume (TSPO VT) in the prefrontal cortex and the anterior cingulate cortex.
Patients with high levels of TSPO showed greater reductions in depression ratings over the course of the study than those with normal levels of TSPO at baseline.
Attwells and colleagues conclude that “this personalized medicine approach of matching a marker of gliosis to [an anti-inflammatory treatment] …should be applied in early development of novel therapeutics, in particular for [treatment-resistant depression].”
Editor’s Note: These findings are of considerable importance, as they are among the first to indicate that measures of inflammation may predict response to an anti-inflammatory medication such as celecoxib. In a 2013 article in the journal JAMA Psychiatry, Charles L. Raison and colleagues reported that patients with high levels of the peripheral inflammatory marker CRP saw marked improvement in their depression when they received the anti-inflammatory treatment infliximab while those with lower or normal levels of inflammation actually worsened.
Quetiapine Reduced Childhood Mania, Especially in Those with Thicker Frontal Temporal Regions
 In a symposium at the 2019 meeting of the American Academy of Child and Adolescent Psychiatry, researcher Melissa P. Delbello reported that six weeks of treatment with either lithium or quetiapine was effective in childhood mania, but quetiapine had a higher response rate of 71% versus 46% for lithium. Delbello found two types of structural changes on functional magnetic resonance imaging (fMRI). Some children had thicker frontal temporal regions, while others had thinning in these areas. The first group of patients had a 100% response to quetiapine, but only 53% of the second group responded to quetiapine.
In a symposium at the 2019 meeting of the American Academy of Child and Adolescent Psychiatry, researcher Melissa P. Delbello reported that six weeks of treatment with either lithium or quetiapine was effective in childhood mania, but quetiapine had a higher response rate of 71% versus 46% for lithium. Delbello found two types of structural changes on functional magnetic resonance imaging (fMRI). Some children had thicker frontal temporal regions, while others had thinning in these areas. The first group of patients had a 100% response to quetiapine, but only 53% of the second group responded to quetiapine.
In contrast, other researchers have found lithium superior to quetiapine. Vivian Kafantaris showed that patients who respond well to lithium show improvements in white matter abnormalities. Michael Berk and colleagues found that a year on lithium was superior to quetiapine on all measures including cognition and brain imaging in patients having their first episode of mania.
Inflammation Predicts Lower Frontal and Temporal White Matter Volumes in Early-Stage Bipolar Disorder
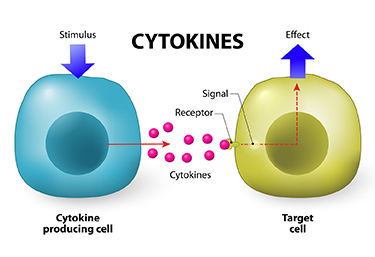 At the 2019 meeting of the International Society for Bipolar Disorders, researcher David Bond found that seven inflammatory cytokines predicted lower white matter volumes in the left frontal and bilateral temporal lobes, as well as in the cingulate and inferior frontal gyri. Cytokines are secreted by some immune cells and send signals that can produce an effect in other cells.
At the 2019 meeting of the International Society for Bipolar Disorders, researcher David Bond found that seven inflammatory cytokines predicted lower white matter volumes in the left frontal and bilateral temporal lobes, as well as in the cingulate and inferior frontal gyri. Cytokines are secreted by some immune cells and send signals that can produce an effect in other cells.
Bond noted that greater inflammation did not predict lower parietal or occipital white matter volumes, suggesting that inflammation had a greater effect on white matter volume in those parts of the brain most closely linked to mood disorders.
Lithium Reverses Thinning of the Cortex That Occurs in Bipolar Disorder
In a 2018 article in the journal Molecular Psychiatry, researcher Derrek P. Hibar reported findings from the largest study to date of cortical gray matter thickness. Researchers in the ENIGMA Bipolar Disorder Working Group, which comprises 28 international research groups, contributed brain magnetic resonance imaging (MRI) from 1837 adults with bipolar disorder and 2582 healthy control participants.
Hibar and colleagues in the working group found that in adults with bipolar disorder, cortical gray matter was thinner in the frontal, temporal, and parietal regions of both brain hemispheres. They also found that bipolar disorder had the strongest effect on three regions in the left hemisphere: the pars opercularis, the fusiform gyrus, and the rostral middle frontal cortex.
Those who had had bipolar disorder longer (after accounting for age at the time of the MRI) had less cortical thickness in the frontal, medial parietal, and occipital regions.
A history of psychosis was associated with reduced surface area.
The researchers reported the effects of various drug treatment types on cortical thickness and surface area. In adults and adolescents, lithium was associated with improvements in cortical thickness, and the researchers hypothesized that lithium’s protective effect on gray matter was responsible for this finding. Antipsychotics were associated with decreased cortical thickness.
In people taking anticonvulsant treatments, the thinnest parts of the cortex were the areas responsibly for visual processing. Visual deficits are sometimes reported in people taking anticonvulsive treatments.
Lithium Reverses Some White Matter Abnormalities in Youth with Bipolar Disorder
 Multiple groups of researchers have reported the presence of white matter tract abnormalities in patients with bipolar disorder. Some of these abnormalities correlate with the degree of cognitive dysfunction in these patients. These white matter tract abnormalities, which are measured with diffusion tensor imaging (DTI), are widespread and can appear as early as childhood in people with bipolar disorder. Researcher Vivian Kafantaris mentioned at the 2019 meeting of the International Society for Bipolar Disorders that lithium treatment in children and adolescents normalizes these alterations, as described in an article she and her colleagues published in the journal Bipolar Disorders in 2017.
Multiple groups of researchers have reported the presence of white matter tract abnormalities in patients with bipolar disorder. Some of these abnormalities correlate with the degree of cognitive dysfunction in these patients. These white matter tract abnormalities, which are measured with diffusion tensor imaging (DTI), are widespread and can appear as early as childhood in people with bipolar disorder. Researcher Vivian Kafantaris mentioned at the 2019 meeting of the International Society for Bipolar Disorders that lithium treatment in children and adolescents normalizes these alterations, as described in an article she and her colleagues published in the journal Bipolar Disorders in 2017.
Editor’s Note: This is another reason to consider the use of lithium in children with bipolar disorder. Lithium treatment may help normalize some of the earliest signs of neuropathology in the illness.
Inflammation Associated With Duration of Untreated Unipolar Depression
Researcher Sophia Attwells and colleagues reported at a 2018 scientific meeting that the longer the time that a patient went without treatment for depression, the more inflammation they exhibited on positron emission tomography (PET) scans. Attwells and colleagues used the PET scans to assess the total distribution volume of TSPO, which is a marker of brain microglial activation, a form of inflammation.
Strikingly, in participants who had untreated major depressive disorder for 10 years or longer, TSPO distribution volume was 29–33% greater in the prefrontal cortex, anterior cingulate cortex, and insula than in participants who were untreated for 9 years or less. TSPO distribution volume was 31–39% greater in these three important regions of gray matter in participants with long durations of untreated major depressive disorder than in healthy control participants.
Editor’s Note: In schizophrenia, the duration of untreated interval (DUI) is associated with a poor prognosis, but not with inflammation. Researcher Yvette Sheline has also reported that less time on antidepressants compared to more time treated with them was associated with greater hippocampal volume loss with aging in patients with major depression.
Given Attwells and colleagues’ remarkable finding about the adverse effects of the DUI in depression, including inflammation and brain volume loss, and other findings that associate more episodes with poorer functioning, cognition, and treatment responsiveness, physicians and patients should think hard about committing to long-term antidepressant treatment to prevent episodes, beginning early in the course of illness.
This editor (Robert M. Post) would propose that if a second depressive episode occurs after a first depression that responded well to treatment, this would be an appropriate time to start antidepressant prophylaxis. Most guidelines suggest that prophylaxis be started after a third episode, but these recommendations generally do not account for newer data on the pernicious effects of experiencing repeated depressive episodes. In addition to causing dysfunction and disability, going through four depressive episodes doubles the risk of dementia in old age, and this risk increases further with each successive episode, according to researcher Lars Kessing.
Having too many depressions is bad for the brain. In Kessing’s studies, two episodes of unipolar or bipolar depression did not increase the risk of dementia compared to the general population, while four depressions did. One could compare the effects of repeated depressions on the brain to the effects of heart attacks on the heart muscle. A heart might still function well after one or even two heart attacks, but the chances of significant loss of function and the risk of congestive heart failure increase as a function of the number of heart attacks. After even one heart attack, most patients change their lifestyle and/or go on prophylactic medications to reduce risk factors such as elevated blood pressure, cholesterol, triglycerides, weight, blood sugar, and smoking. The benefits of reducing heart attacks are a no brainer. Trying to prevent recurrent depression with pharmacotherapy and adjunctive psychotherapy after a second depressive episode should be a no brainer too.
In addition, if antidepressants are not effective enough in preventing depressions, lithium is an option, even in unipolar depression, for preventing both episodes and suicide. The evidence of efficacy in both instances is very strong according to an article by Mohammed T. Abou-Saleh in the International Journal of Bipolar Disorders in 2017. The renowned psychiatrist Jules Angst’s recommendation as to when to start lithium treatment was that if a patient had had one episode or more in the previous five years in addition to the present episode, then they were likely to have two further episodes in the following five years, and lithium prophylaxis would be recommended.
Risk Gene for Bipolar Disorder Implicated in Depressed Behaviors and Abnormal Firing of GABA Neurons
At a 2018 scientific meeting and in a 2017 article in the journal PNAS, researcher Shanshan Zhu and colleagues reported that mice genetically engineered to lack the protein Ankyrin-G in certain neurons showed increases in depression- and mania-like behavior after being exposed to defeat stress (by repeatedly being placed in physical proximity to a larger, more aggressive mouse), which is often used to model human depression.
The researchers targeted the gene ANK3, which is responsible for the production of Ankyrin-G, and has been linked to bipolar disorder in genome-wide association studies. By manipulating the gene, they could eliminate Ankyrin-G in pyramidal neurons in the forebrain, a region relevant to many psychiatric disorders. Pyramidal neurons perform key brain functions, sending nerve pulses that lead to movement and cognition.
The missing Ankyrin-G affected sodium channels (which allow for the flow of sodium ions in and out of cells) and potassium channels. The neurochemical GABA (which typically inhibits nerve impulses) was also dysregulated, resulting in the kind of disinhibition seen in psychosis. Mice showed dramatic behavioral changes ranging from hyperactivity to depression-like behavior (e.g. giving up in a forced swimming test). The hyperactivity decreased when the mice were given treatments for human mania, lithium or valproic acid.
While mutations in the ANK3 gene may disturb sodium channels, another gene linked to depression and bipolar disorder, CACNA1C, affects calcium channels.
In a related study by researcher Rene Caballero-Florán and colleagues that was also presented at the meeting, mice were genetically engineered in such a way that interactions between Ankyrin-G and GABA Type A Receptor-Associated Protein (GABARAP) were disrupted, leading to deficits in inhibitory signaling. These deficits were partially corrected when the mice were treated with lithium.
The study by Caballero-Florán and colleagues used mice with a mutation known as W1989R in the ANK3 gene. Through a program that examines the genes of people with bipolar disorder, the researchers also identified a family with this genetic mutation, including a patient with type I bipolar disorder with recurrent mania and depression who has responded well to lithium treatment.
White Matter Abnormalities in Obesity
Researcher Ramiro Reckziegel and colleagues reported at a recent scientific meeting that white matter is abnormal in obese adults with bipolar disorder. In a 2018 article in the journal Schizophrenia Bulletin, Reckziegel reported that body mass index (BMI) was associated with reduced fractional anisotropy, a measure of brain fiber integrity, in the cingulate gyrus in patients with bipolar disorder. This finding implies that obesity may play a role in white matter microstructure damage in the limbic system.