Psychotherapy Prevents Recurrence of Depression
New research shows that psychotherapy lowers the risk of relapse in unipolar major depression more than “treatment as usual” does, and also heads off depression in children at high risk.
At the 2013 meeting of the American Psychiatric Association, researcher Pim Cuijpers reviewed 32 trials of cognitive behavior therapy, intensive behavioral therapy, and problem solving therapy used for the prevention of depression and found that these therapies were associated with a 21% lower risk of relapse compared to treatment as usual.
There were five critical elements that made these therapies useful: they supported coping with depression, and they included exercise, mindfulness, internet-based cognitive behavior therapy, and problem solving.
Among those who presented at the meeting, Greg Clarke of Kaiser Permanente, Oregon discussed an 8-week course on coping with stress given to a group of adolescents (aged 14 to 16) who had four times the normal risk of developing depression because each had a parent with depression. Clarke found a significant reduction in depression among the adolescents who received therapy compared to controls.
Insomnia can be a precursor to a first depression or to recurrent depression. Cognitive behavior therapy was more effective in improving sleep than a comparative sleep hygiene course.
Researcher Judy Garber presented data showing that cognitive behavior therapy was effective in 13- to 17-year-olds who had a parent with depression and had themselves had a prior depression or were currently sub-syndromal. The effect of the therapy was only significant if the parent was not depressed at intake.
New Drug for Insomnia
The Federal Drug Administration (FDA) review committee suggested that a new type of drug for the treatment of insomnia might be approved in the future if the company who produces the drug, Merck, begins manufacturing it in smaller doses.
The drug, suvorexant, works differently from most sleep medications. Instead of creating sleepiness, it blocks a type of neurotransmitters called orexins, which are responsible for wakefulness. (Suvorexant is a selective antagonist of orexin receptors OX1R and OX2R and blocks the binding of orexin A and B to these receptors, presumably inhibiting activation of neurons of the arousal system.) The drug has a 12-hour half-life, longer than other insomnia drugs, thus morning carryover sedation is a potential problem.
The FDA asked that suvorexant be produced at the lower dose of 10mg, with the option of prescribing it at higher doses (15mg to 20mg) if a patient tolerated the 10mg dose and that dose did not sufficiently improve the patient’s insomnia. Merck had initially proposed doses of 15mg to 40mg.
Drugs that Antagonize the Glutamate NMDA Receptor are a New Approach to Antidepressant Treatment
 Several antidepressant drugs work by blocking activity at the NMDA receptor for the excitatory neurotransmitter glutamate. These drugs include intravenous ketamine, a potent NMDA receptor blocker that can produce antidepressant effects within 2 hours of administration, and memantine (Namenda), which is less potent, acts more slowly, and can potentiate the antidepressant effects of lamotrigine and help stabilize mood in patients with treatment-resistant bipolar disorder. Now a drug with a related mechanism, D-cycloserine, has been shown to have antidepressant effects. High doses of the drug act as an antagonist at the glycine site of the NMDA receptor, blocking glycine’s ability to facilitate glutamate transmission through the receptor.
Several antidepressant drugs work by blocking activity at the NMDA receptor for the excitatory neurotransmitter glutamate. These drugs include intravenous ketamine, a potent NMDA receptor blocker that can produce antidepressant effects within 2 hours of administration, and memantine (Namenda), which is less potent, acts more slowly, and can potentiate the antidepressant effects of lamotrigine and help stabilize mood in patients with treatment-resistant bipolar disorder. Now a drug with a related mechanism, D-cycloserine, has been shown to have antidepressant effects. High doses of the drug act as an antagonist at the glycine site of the NMDA receptor, blocking glycine’s ability to facilitate glutamate transmission through the receptor.
Glutamate is the major excitatory neurotransmitter in the brain and is important for the development of long-term memory. However, glutamate overactivity may contribute to depression. Decreasing this overactivity with the drugs noted above appears to produce antidepressant effects.
Uriel Heresco-Levy et al. reported in a 2013 article in the Journal of Neuropsychopharmacology that high doses of D-cycloserine (1000mg/day) had substantially greater antidepressant effects than placebo in a study of 26 patients with treatment-resistant depression. The drug was well tolerated.
Interestingly, low doses of the same drug have a different effect, acting as a partial agonist at the same site, facilitating glutamate transmission and enhancing the new learning that is necessary in cognitive behavioral therapy for anxiety disorders.
Editor’s Note: D-cycloserine requires further study in larger controlled trials, but this small study suggests promise. While ketamine’s effects are rapid in onset, they are also difficult to sustain. This study suggests a possible route to a slower onset with longer-lasting antidepressant effects.
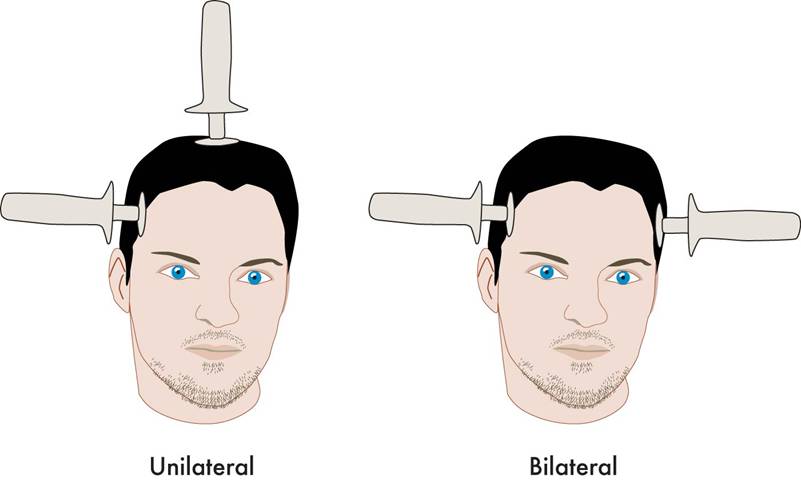
Effectiveness of Continuation Right Unilateral ECT Plus Pharmacotherapy Compared to Pharmacotherapy Alone
A new study is one of the first to find that after successful electroconvulsive therapy (ECT) treatment for depression, continuation of ECT together with pharmacotherapy was superior to continuation with pharmacotherapy alone. ECT produces a seizure while a patient is under anesthesia. The treatment has been successful in acutely treating many patients with severe depression who have not responded to other treatments. The question remains how to maintain the positive acute effects of ECT for a longer duration.
The new research was published in the Journal of ECT in 2013 by Axel Nordenskjöld et al. Patients with unipolar or bipolar depression who improved after an acute series of ECT (usually given 3 times per week) were randomized to receive either continuing ECT plus pharmacotherapy or pharmacotherapy alone. The ECT continuation group received weekly ECT for 6 weeks and every 2 weeks thereafter for a total of 29 ECT treatments in one year. The pharmacotherapy consisted of antidepressants (98%), lithium (56%), and antipsychotics (30%). Venlafaxine (Effexor) was considered the first choice for medication, and lithium augmentation was offered to all patients (not just those with bipolar depression). Of the participants, 64% had recurrent unipolar depression, while less than 20% had bipolar depression.
Among all the patients who were randomized at the beginning of the study (the intent-to-treat cohort), the one-year relapse rate was 61% for patients treated with pharmacotherapy alone and 32% for patients treated with the ECT plus pharmacotherapy (p=0.036). Relapse rates at 6 months were 54% for the pharmacotherapy alone group and 29% for the group receiving ECT plus pharmacotherapy. Some patients required inpatient care during the trial—36% of the patients in the pharmacotherapy alone group and 20% of those in the pharmacotherapy plus ECT group. There was no evidence of a differential effect on cognition across the two groups. (There is concern that bilateral ECT can adversely affect cognition, especially autobiographical memory, but this is not a concern with right unilateral ECT.)
Various parameters for ECT have been studied. This research used unilateral ultrabrief pulse ECT. These parameters are standard in Sweden, where the study took place, and these results are of particular interest as they differ from a US study that used bilateral ECT with treatments given more intermittently. In that study, in which response to ECT with pharmacotherapy did not differ from response to pharmacotherapy alone, ECT continuation was given in the form of 4 weekly treatments, 4 biweekly treatments and then 2 monthly treatments, and this regimen resulted in a relapse rate of 37% within 6 months (Kellner et al. 2006).
Only one other study in geriatric patients who were psychotically depressed showed superiority of continuation ECT.
Editor’s Note: The take-home message from this study may be that for patients with recurrent unipolar depression who respond positively to a course of right unilateral ultrabrief pulse ECT, continuation of regular ECT plus pharmacotherapy is worth considering over pharmacotherapy alone. While direct comparative studies have not been performed, it would appear reasonable to use weekly ECT for 6 weeks and then every 2 weeks thereafter for the continuation ECT treatment rather than a more intermittent series of treatments as in the studies of Kellner et al. Previous studies have shown that the addition of lithium to antidepressants is superior to antidepressants alone in the continuation phase of ECT (Sackeim et al. 2001), so having lithium in the regimen would also appear useful.
A New Target for Deep Brain Stimulation (DBS) for Refractory Depression
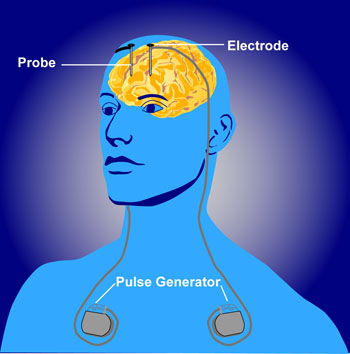 Deep brain stimulation (DBS) is a treatment for illnesses such as Parkinson’s, chronic pain, and depression. In DBS, electrodes inserted in the brain produce electrical impulses. Different anatomical parts of the brain have been targeted successfully using DBS. These include the subgenual anterior cingulate (Area 25), the nucleus accumbens, and the habenula. Now researcher Thomas E. Schlaepfer has found that inserting the n. accumbens electrode deeper and closer to the ventral tegmental area or the VTA (which contains dopamine cell bodies) produces more rapid onset of antidepressant effects (in a matter of days) in a high percentage of patients (7 out of 8 according to one of Schlaepfer’s reports). Stimulation of the nucleus accumbens in the typical location resulted in 50% improvement in 5 out of 10 patients after one year of follow-up.
Deep brain stimulation (DBS) is a treatment for illnesses such as Parkinson’s, chronic pain, and depression. In DBS, electrodes inserted in the brain produce electrical impulses. Different anatomical parts of the brain have been targeted successfully using DBS. These include the subgenual anterior cingulate (Area 25), the nucleus accumbens, and the habenula. Now researcher Thomas E. Schlaepfer has found that inserting the n. accumbens electrode deeper and closer to the ventral tegmental area or the VTA (which contains dopamine cell bodies) produces more rapid onset of antidepressant effects (in a matter of days) in a high percentage of patients (7 out of 8 according to one of Schlaepfer’s reports). Stimulation of the nucleus accumbens in the typical location resulted in 50% improvement in 5 out of 10 patients after one year of follow-up.
Schlaepfer believes that changing the position of the electrode places it in the medial forebrain bundle (MFB), which contains fibers going from the VTA to the nucleus accumbens. Interestingly, when animals are given the opportunity to self-stimulate this area (by pressing a lever that activates an electrode placed in the area), they will, indicating it is rewarding or pleasurable. The same animals will avoid self-stimulating certain other fiber tract sites in the brain.
Schlaepfer’s research was presented at the 2013 meeting of the Society of Biological Psychiatry, and the abstract is available in the Convention Supplement to the journal Biological Psychiatry, Volume 73, Number 9S.
VNS Covered For Reimbursement For Refractory Epilepsy But Not Refractory Depression
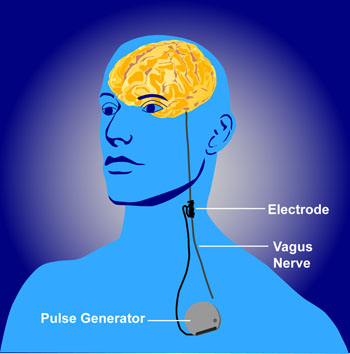 The federal Centers for Medicare and Medicaid Services (CMS) appear to discriminate against people with psychiatric illness. They will not approve the use of VNS (Vagus Nerve Stimulation) for treatment resistant depression among Medicare beneficiaries but have approved it for refractory epilepsy.
The federal Centers for Medicare and Medicaid Services (CMS) appear to discriminate against people with psychiatric illness. They will not approve the use of VNS (Vagus Nerve Stimulation) for treatment resistant depression among Medicare beneficiaries but have approved it for refractory epilepsy.
Vagus nerve stimulation consists of electrical stimulation of the vagus, one of the cranial nerves. The Federal Drug Administration (FDA) has already approved VNS for both illnesses, meaning that it has been judged to be effective and safe in both.
The CMS decision will make it harder for poor patients to access this treatment because it means that Medicare will not reimburse the cost of the treatment.
Cyberonics, the company that manufactures the device used to provide VNS, applied for CMS approval of the use of VNS for depression in 2007 and was rejected, but hoped that the research collected in the intervening years that shows VNS’s efficacy in treatment-resistant depression would be enough to get eventual approval from CMS. They have been rejected again.
Editor’s Note: The data supporting VNS’s efficacy in epilepsy are skimpy and without a large effect size, so it cannot be argued that the quality of data supporting the coverage of VNS for depression is the reason it hasn’t been approved for that purpose. Both epilepsy and depression can be devastating and life altering. To support the use and reimbursement for the same FDA-approved device for one but not the other illness seems worthy of protest.
This appears to be another example of the incongruous use of FDA approval status to deny coverage. Insurance companies and government agencies routinely use lack of FDA approval to deny reimbursement claims for many commonly used drugs and treatments, while at the same time, in cases such as the use of VNS, actual FDA approval is disregarded and considered an insufficient criterion. Having it both ways does not seem to bother the policy makers who appear to put the financial bottom line ahead of ethics, fairness, and good medical care.
RTMS in Depression: Positive Effects in Long-Term Follow-Up
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive treatment that uses a rapidly changing magnetic field to target neurons, creating a weak electric current. It is used to treat depression, strokes, and other neurological and psychiatric conditions. At the 2013 meeting of the American Psychiatic Association (APA), researcher Linda L. Carpenter reported on new findings about the long-term effects of rTMS.
In the new research, which was led by Mark Andrew Demitrack, 307 patients with unipolar depression who had not responded well to previous antidepressant treatment were given rTMS. They were treated in 43 different clinical practices and administered rTMS according to their evaluating physician, following FDA guidelines. Of the 307 patients who began the study, 264 benefited from initial treatments with rTMS, were tapered off rTMS, and agreed to participate in a one-year follow-up period, by the end of which 68% had improved and 40.4% had achieved complete remission as measured by the Clinical Global Impressions scale for severity of illness.
However, some study participants (30.2%) had worsened by the first follow-up assessment at the 3-month mark, and rTMS had to be re-introduced.