Risk Gene for Bipolar Disorder Implicated in Depressed Behaviors and Abnormal Firing of GABA Neurons
At a 2018 scientific meeting and in a 2017 article in the journal PNAS, researcher Shanshan Zhu and colleagues reported that mice genetically engineered to lack the protein Ankyrin-G in certain neurons showed increases in depression- and mania-like behavior after being exposed to defeat stress (by repeatedly being placed in physical proximity to a larger, more aggressive mouse), which is often used to model human depression.
The researchers targeted the gene ANK3, which is responsible for the production of Ankyrin-G, and has been linked to bipolar disorder in genome-wide association studies. By manipulating the gene, they could eliminate Ankyrin-G in pyramidal neurons in the forebrain, a region relevant to many psychiatric disorders. Pyramidal neurons perform key brain functions, sending nerve pulses that lead to movement and cognition.
The missing Ankyrin-G affected sodium channels (which allow for the flow of sodium ions in and out of cells) and potassium channels. The neurochemical GABA (which typically inhibits nerve impulses) was also dysregulated, resulting in the kind of disinhibition seen in psychosis. Mice showed dramatic behavioral changes ranging from hyperactivity to depression-like behavior (e.g. giving up in a forced swimming test). The hyperactivity decreased when the mice were given treatments for human mania, lithium or valproic acid.
While mutations in the ANK3 gene may disturb sodium channels, another gene linked to depression and bipolar disorder, CACNA1C, affects calcium channels.
In a related study by researcher Rene Caballero-Florán and colleagues that was also presented at the meeting, mice were genetically engineered in such a way that interactions between Ankyrin-G and GABA Type A Receptor-Associated Protein (GABARAP) were disrupted, leading to deficits in inhibitory signaling. These deficits were partially corrected when the mice were treated with lithium.
The study by Caballero-Florán and colleagues used mice with a mutation known as W1989R in the ANK3 gene. Through a program that examines the genes of people with bipolar disorder, the researchers also identified a family with this genetic mutation, including a patient with type I bipolar disorder with recurrent mania and depression who has responded well to lithium treatment.
Specific Probiotics Reduce Re-Hospitalizations for Bipolar Disorder
In a 2018 article published in the journal Bipolar Disorders, researcher Faith Dickerson and colleagues reported that in a small study of 66 people who had been hospitalized for mania, taking specific probiotic supplements upon their release reduced re-hospitalizations compared to taking placebo.
The study followed patients for 24 weeks after their hospitalization. They were randomized to receive either the combination of Lactobacillus rhamnosus strain GG and Bifidobacterium animalis subsp. lactis strain Bb12 or placebo in addition to their regular medications. While 17 of the 33 participants in the placebo group (51.5%) had at least one re-hospitalization during the study period, only eight (24.2%) of the participants taking probiotics had a re-hospitalization. The duration of the re-hospitalizations was also shorter for those taking probiotics (2.8 days on average versus 8.3 days for those taking placebo).
In a personal communication to this editor (Robert M. Post), Chris Aiken, Instructor in Clinical Psychiatry at Wake Forest University School of Medicine, who was not involved in the study, provided some clarifying details to this editor about the use of probiotics to reduce manic relapse. Aiken explained, “Apparently, it’s important to get both the right species (e.g. Bifidobacterium lactis) and the right strain (e.g. Bb-12) in choosing a probiotic. The study mentions that one of the strains (Bb-12) is patented and only available in Europe, but it has been licensed to a few U.S. companies.
“I found two products that contain the exact strains in the study and wrote this up for patients: In [the] study [noted above], a probiotic capsule containing Bifidobacterium lactis Bb-12 and Lactobacillus rhamnosus GG lowered the risk of psychiatric hospitalization threefold. [Both] strains are available in the supplement Emergen-C and in a liquid probiotic designed for infants, Culturelle Baby Grow and Thrive. The infant serving would suffice for adults as well. You could also get the two strains by combining two separate probiotic capsules: Align Daily Immune Support and Culturelle Digestive Health Daily Priobiotic.”
Editor’s Note: We are grateful to Dr. Aiken for this added information. We also found that USANA probiotic also contains both strains used in the study. Recent research has found more and more connections between inflammatory processes and mental health. This study contributes to our understanding of the connection between gut health and the brain.
Treatment Approaches to Childhood-Onset Treatment-Resistant Bipolar Disorder
Dear readers interested in the treatment of young children with bipolar disorder and multiple other symptoms: In 2017, BNN Editor Robert M. Post and colleagues published an open access paper in the journal The Primary Care Companion for CNS Disorders titled “A Multi-Symptomatic Child: How to Track and Sequence Treatment.” The article describes a single case of childhood-onset bipolar disorder shared with us via our Child Network, a research program in which parents can create weekly ratings of their children’s mood and behavioral symptoms, and share the long-term results in graphic form with their children’s physicians.
Here we summarize potential treatment approaches for this child, which may be of use to other children with similar symptoms.
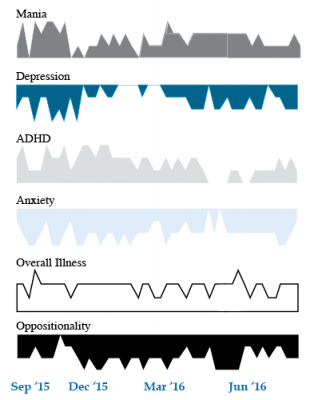
We present a 9-year-old girl whose symptoms of depression, anxiety, attention-deficit hyperactivity disorder (ADHD), oppositional behavior, and mania were rated on a weekly basis in the Child Network under a protocol approved by the Johns Hopkins School of Medicine Institutional Review Board. The girl, whose symptoms were rated consistently for almost one year, remained inadequately responsive to lithium, risperidone, and several other medications. We describe a range of other treatment options that could be introduced. The references for the suggestions are available in the full manuscript cited above, and many quotes from the original article are reprinted here directly.
As illustrated in the figure below, after many weeks of severe mania, depression, and ADHD, the child initially appeared to improve with the introduction of 4,800 micrograms per day of lithium orotate (a more potent alternative to lithium carbonate that is marketed as a dietary supplement), in combination with 1 mg per day of guanfacine, and 1 mg per day of melatonin.
Despite continued treatment with lithium orotate (up to 9,800 micrograms twice per day), the patient’s oppositional behavior worsened during the period from November 2015 to March 2016, and moderate depression re-emerged in April 2016. Anxiety was also generally less severe from December 2015 to July 2016, and weekly ratings of overall illness remained in the moderate severity range (not illustrated).
In June 2016, the patient began taking risperidone (maximum dose 1.7 mg/day) instead of lithium, and her mania improved from moderate to mild. There was little change in her moderate but fluctuating depression ratings, but her ADHD symptoms got worse.
The patient had been previously diagnosed with bipolar II disorder and anxiety disorders including school phobia, generalized anxiety disorder, and obsessive compulsive disorder.
Given the six weeks of moderate to severe mania that the patient experienced in October and November 2015, she would meet criteria for a diagnosis of bipolar I disorder.
Targeting Symptoms to Achieve Remission
General treatment goals would include: mood stabilization prior to use of ADHD medications, a drug regimen that maximizes tolerability and safety, targeting of residual symptoms with appropriate medications supplemented with nutraceuticals, recognition that complex combination treatment may be necessary, and combined use of medications, family education, and therapy.
Mood Stabilizers and Atypical Antipsychotics to Maximize Antimanic Effects
None of the treatment options in this section are approved by the US Food and Drug Administration for use in children under 10 years of age, so all of the suggestions are “off label.” Further, they may differ from what other investigators in this area of medicine would suggest, especially since evidence-based medicine’s traditional gold standard of randomized placebo-controlled clinical trials is impossible to apply here, given the lack of research in children with bipolar disorder.
As we share in the original article, reintroducing lithium alongside risperidone could be effective, as “combinations were more effective than monotherapy in a study [by] Geller et al. (2012), especially when they involved an atypical antipsychotic such as risperidone. This might include the switch from lithium orotate to lithium carbonate,” the typical treatment for bipolar disorder, on which more research has been done. “Combinations of lithium and valproate were also more effective than either [drug alone]…in the studies of Findling et al. (2006),” and many patients needed stimulants in addition.
“Most children also needed combinations of mood stabilizers (lithium, carbamazepine, valproate) in the study [by] Kowatch et al. (2000).” In a 2017 study by Berk et al. of patients hospitalized for a first mania, randomization to lithium for one year was more effective than quetiapine on almost all outcome measures.
Targeting ADHD
“[The increased] severity of [the child’s] ADHD despite improving mania speaks to the…utility of adding a stimulant to the regimen that already includes…guanfacine,” which is a common non-stimulant treatment for ADHD. “This would be supported by the data of Scheffer et al. (2005) that stimulant augmentation for residual ADHD symptoms does not [worsen] mania, and that the combination of a stimulant and guanfacine may have more favorable effects than stimulants alone.”
However, the consensus in the field is that mood stabilization should be achieved first, before low to moderate (but not high) doses of stimulants are added. “Thus, in the face of an inadequate response to the lithium-risperidone combination in this child, stimulants could be deferred until better mood stabilization was achieved.”
Other Approaches to Mood Stabilization and Anxiety Reduction
“The anticonvulsant mood stabilizers (carbamazepine, lamotrigine, and valproate) each have considerable mood stabilizing and anti-anxiety effects, at least in adults with bipolar disorder. With inadequate mood stabilization of this patient on lithium and risperidone, we would consider the further addition of lamotrigine.
Lamotrigine appears particularly effective in adults with bipolar disorder who have a personal history and a family history of anxiety (as opposed to mood disorders), and it has positive open data in adolescents with bipolar depression and in a controlled study of maintenance (in teenagers 13–17, but not in preteens 10–12) (Findling et al. 2015). With better mood stabilization, anxiety symptoms usually diminish…, and we would pursue these strategies [instead of using] antidepressants for depression and anxiety in young children with bipolar disorder.”
“Carbamazepine appears to be more effective in adults with bipolar who have [no] family history of mood disorders,” unlike lithium, which seems to work better in people who do have a family history of mood disorders.
“While the overall results of oxcarbazepine in childhood mania were negative, they did exceed placebo in the youngest patients (aged 7–12) as opposed to the older adolescents (13–18) (Wagner et al. 2006).
“There are long-acting preparations of both carbamazepine (Equetro) and oxcarbazepine (Oxtellar) that would allow for all nighttime dosing to help with sleep and reduce daytime side effects and sedation. Although data [on] anti-manic and antidepressant effects in adults are stronger for carbamazepine than oxcarbazepine,” there are good reasons to consider oxcarbazepine. First, there is the finding mentioned above that oxcarbazepine worked best in the youngest children. Second, there is a lower incidence of severe white count suppression on oxcarbazepine. Third, it has less of an effect on liver enzymes than carbamazepine. However, low blood sodium levels are more frequent on oxcarbazepine than carbamazepine.
Other Atypical Antipsychotics That May Improve Depression
FDA Approves Extended-Release Aripiprazole Injected Monthly to Prevent Manic and Mixed Episodes in Bipolar I
In 2017 the US Food and Drug Administration approved a monthly injectable form of the atypical antipsychotic drug aripiprazole, Abilify Maintena, for the prevention of manic and mixed episodes in bipolar I disorder. The intramuscular injections are available for monotherapy in preparations of 300 mg or 400 mg. Maintena did not prevent depressive episodes.
Maintena is already FDA-approved for the treatment of schizophrenia and Tourette’s syndrome in adults.
The approval for bipolar I disorder follows a 52-week phase 3, double-blind, placebo-controlled randomized trial. Participants were experiencing a manic episode during screening for the study, met the criteria for bipolar I disorder, and had had at least one prior manic or mixed episode severe enough to require treatment.
Compared to placebo, Maintena in once-a-month injections delayed the recurrence of any mood episode following the initial manic episode at screening. When the researchers separated their analysis based on type of episode, Maintena reduced manic and mixed episodes compared to placebo, but did not do a better job than placebo at preventing depressive episodes.
An oral antipsychotic must be administered for 14 days following the first injection of Maintena. The extended-release injection is available as 300 mg– or 400 mg–strength powder that may be reconstituted, or as prefilled syringes.
Editor’s Note: Because Maintena is delivered as a once-a-month injection, it may be helpful for patients who struggle to take daily oral medications.
Inflammatory Marker CRP Higher in Bipolar Disorder, Particularly Mania
Inflammation has been linked to mood disorders. A 2016 meta-analysis in the journal Lancet Psychiatry described the role of inflammatory marker C-reactive protein (CRP) in bipolar episodes. Researchers led by Brisa S. Fernandes identified 27 studies that measured CRP levels in a total of 2,161 patients with bipolar disorder and 81,932 healthy participants. The researchers determined that compared to healthy controls, people with bipolar disorder have higher levels of CRP. CRP levels were moderately elevated between episodes and during depression, and substantially elevated during episodes of mania.
Editor’s Note: This meta-analysis shows that CRP is linked to bipolar disorder, and the inflammatory burden is highest during mania. It remains to be seen whether anti-inflammatory treatments work best in patients with high CRP levels compared to normal CRP levels.
CRP is also a risk factor for cardiovascular disease, and lithium and other treatments for bipolar disorder probably lower CRP levels.
The same group of researchers previously showed that statins, drugs typically used to lower cholesterol, could help alleviate depression. Since statins have anti-inflammatory effects, they can probably reduce depression risk in addition to lowering cardiovascular risk, as initial studies suggest.
Other drugs with anti-inflammatory effects that may improve depression include the anti-arthritis drug celecoxib and the antibiotic minocycline. The amino acid N-acetylcysteine and omega-3 fatty acids also have anti-inflammatory effects and have been found to improve depression in some studies.
Preventative Treatment Should Begin After First Manic Episode
Evidence from multiple studies has indicated the importance of beginning preventative treatment, particularly with lithium, early in the course of bipolar disorder. A 2016 comprehensive literature review by researcher Katie Joyce and colleagues in the International Journal of Bipolar Disorders concluded that psychoeducation and medication are more effective in bipolar disorder when applied in earlier stages of the illness rather than later stages.
Several studies suggest that treatment for bipolar disorder should be started specifically after the first manic episode.
A 2014 study by researcher Lars Kessing and colleagues in the British Journal of Psychiatry examined 4,700 patients treated with lithium in Denmark. Kessing and colleagues found that those who started treatment after one manic episode were less likely to find lithium ineffective than those who started later.
Another study by researcher Michael Berk and colleagues presented at the International Society for Bipolar Disorders found that after a first manic episode, a year of treatment with lithium was much more effective on all measures of outcome, including mania and depression ratings, brain imaging, and neuropsychological functioning, than a year when patients were randomized to quetiapine (Seroquel).
Researcher Lakshmi Yatham and colleagues presented research at the International Society for Bipolar Disorders showing that patients recovered from the neuropsychological deficits associated with a first episode of mania if they were well treated and had no further episodes, while those who had new episodes did not return to their baseline capabilities. This suggests that early treatment that prevents future episodes helps maintain a healthy brain.
Kessing and colleagues previously reported in the British Journal of Psychiatry in 2013 that patients randomized to two years of treatment in an outpatient clinic specializing in mood disorders following a first hospitalization for mania had 40% fewer recurrences of bipolar episodes over the next six years than those who received treatment as usual. These data indicate that early treatment, which may include psychotherapy, medications, mood charting (i.e. keeping a daily record of symptoms) and illness education, can improve the long-term course of illness. Lithium is often a key component of such treatment.
Editor’s Note: This type of intensive, ongoing treatment is not the norm after a first manic hospitalization in the United States, but it should be. Given the new data on the impact of starting lithium after a first episode of mania, and lithium’s superiority over quetiapine in the year following a first episode, lithium treatment should be standard following a diagnosis of bipolar disorder.
In the past, sometimes doctors have recommended waiting until a patient has had multiple episodes of mania before beginning preventative treatment with lithium. This now appears to be a mistake.
Lithium protects against depressive as well as manic recurrences, and there is also evidence that it increases hippocampal and cortical volume, helping prevent cognitive deterioration in those with mild cognitive impairment. Lithium is also the most effective mood stabilizer for preventing suicide, and it increases the length of telomeres (caps on the end of DNA strands), thus preventing a wide range of medical and psychiatric illnesses. Lithium may need to be combined with other drugs to achieve a complete remission, but using it after a first mania is a good place to start.
Lithium Treatment Reduces Inflammation, Mania-Like Behavior in Rats
Patients with bipolar disorder often show increases in signs of inflammation, including levels of the proteins IL-2, IL-4, Il-6, IL-10 and tumor necrosis factor in their blood. Lithium is the most effective treatment for bipolar disorder, but it is not yet clear how it works. A recent study by researcher Joao de Quevado and colleagues determined that lithium can reduce the same inflammatory markers in rats.
Rats were treated with amphetamine to induce mania-like behavior, which was accompanied by increases in some of the same inflammatory markers in the blood and brain that are increased in people with bipolar disorder. Lithium treatment reduced both the manic behavior and levels of these inflammatory proteins in the rats.
The researchers concluded that lithium may treat mania by reducing inflammation.
Anxiety, Depression, Unstable Mood, and Low-Level Mania Best Predictors of Bipolar Disorder
Researchers are looking for better ways of predicting whether children at risk for bipolar disorder will go on to develop the illness. A 2015 study by David Axelson and colleagues in the American Journal of Psychiatry reported that in the offspring of parents with bipolar disorder, diagnoses of sub-threshold mania, depression, and disruptive behavior disorders were associated with subsequent diagnosis of full-blown Bipolar I or Bipolar II disorders six to seven years later.
More recently, in an article by Danella M. Hafeman and colleagues in the American Journal of Psychiatry, the same group of investigators has examined how symptoms (rather than categorical diagnoses, as in the earlier study) predict the development of bipolar disorder. In children and adolescents at high risk for bipolar disorder (because they have a parent with the disorder) three types of symptoms were the best predictors of later bipolar disorder: anxiety/depression at the time participants entered the study, unstable mood or irritability both when entering the study and shortly before a bipolar diagnosis, and low-level manic symptoms observed shortly before diagnosis.
The earlier the age at which a parent was diagnosed with a mood disorder, the greater the risk that the offspring would also be diagnosed with bipolar disorder. Youth with all four risk factors (anxiety or depression, mood changes, low-level mania, and a parent who was diagnosed with a mood disorder at an early age) had a 49 percent chance of developing bipolar disorder, compared to a 2 percent chance among those without those risk factors.
Childhood onset of bipolar disorder and long delays until first treatment for depression or mania are both significant predictors of a poor outcome in adulthood compared to adult onsets and shorter delays to treatment. Read more
Vitamin D3 Reduces Symptoms of Bipolar Spectrum Disorders
 Vitamin D3 tends to be low in children and adolescents with mania, but supplements may help. In a small open study published in the Journal of Child and Adolescent Psychopharmacology in 2015, Elif M. Sikoglu and colleagues administered 2000 IU of vitamin D3 per day to youth aged 6–17 for eight weeks. Sixteen of the participants had bipolar spectrum disorders (including subthreshold symptoms) and were exhibiting symptoms of mania. Nineteen participants were typically developing youth.
Vitamin D3 tends to be low in children and adolescents with mania, but supplements may help. In a small open study published in the Journal of Child and Adolescent Psychopharmacology in 2015, Elif M. Sikoglu and colleagues administered 2000 IU of vitamin D3 per day to youth aged 6–17 for eight weeks. Sixteen of the participants had bipolar spectrum disorders (including subthreshold symptoms) and were exhibiting symptoms of mania. Nineteen participants were typically developing youth.
At the beginning of the study, the youth with bipolar spectrum disorders had lower levels of the neurotransmitter GABA in the anterior cingulate cortex than did the typically developing youth. Following the eight weeks of vitamin D3 supplementation, mania and depression symptoms both decreased in the youth with bipolar spectrum disorders, and GABA in the anterior cingulate cortex increased in these participants.
Editor’s Note: GABA dysfunction has been implicated in the manic phase of bipolar disorder. While larger controlled studies of vitamin D supplementation are needed, given the high incidence of vitamin D deficiency in youth in the US, testing and treating these deficiencies is important, especially among kids with symptoms of bipolar illness.
Successful Double-Blind Placebo-Controlled Study of Lithium for Acute Mania in Kids 7–17
Lithium is the treatment of choice for adults with bipolar disorder, but has rarely been studied in children or adolescents. One of the first double-blind placebo-controlled trials of lithium for the treatment of mania in children and teens aged 7–17 showed that the drug produced greater improvement in mania than did placebo. Side effects included blurred vision, abdominal pain, diarrhea, nausea, vomiting, fatigue, thirst, increased thyroid-stimulating hormone, decreased appetite, dizziness, sedation, tremor, increased urination, and rash.
In the study by researcher Adelaide S. Robb and colleagues, which was presented at the 2015 meeting of the American Academy of Child and Adolescent Psychiatry, doses began at 300mg twice a day, were based on each child’s weight, and were slowly increased.
At the same meeting, researcher Russell Scheffer presented data on 41 children who continued lithium treatment for 16 weeks with good results. The mean dose was 27.8 +/- 6.7 mg/kg per day.