Multiple Risks of Benzodiazepine Use
Benzodiazepines are a class of drugs that became widely used in the 1970s for their ability to reduce panic, anxiety, and insomnia. Some also functioned as anticonvulsants, reducing seizures. They are considered “downers,” with sedating qualities.
New research shows that benzodiazepine use, particularly long-term use, comes with risks such as increased mortality and mood instability.
At a 2015 scientific meeting, researcher Jari Tiihonen reported that among 21,492 patients with schizophrenia in Sweden, benzodiazepine use was associated with increased mortality, while antidepressant and antipsychotic use decreased mortality.
At the same meeting, researcher Cristina Albott reported that benzodiazepines may interfere with the rapid onset of antidepressant effects usually brought about by intravenous treatment with the drug ketamine.
In 2010, researcher Roy Perlis reported in the Journal of Clinical Psychiatry that in STEP-BD, a large study of people with bipolar disorder, benzodiazepine use was associated with an increased risk of recurrence of mood episodes.
Editor’s Note: Benzodiazepines can also exacerbate symptoms of post-traumatic stress disorder (PTSD) and regular use can lead to a decrease in lifespan. It now seems as though there are many reasons to exercise caution in the use of these drugs.
Very Low Doses of Opioid Buprenorphine May Reduce Suicidal Ideation
There is no perfect treatment to reduce the risk of suicide in someone who is considering it. Antidepressants can reduce suicidal ideation, but they take several weeks to start working. Intravenous ketamine is used at higher doses as an anesthetic, but in low doses works quickly to reduce suicidal thoughts. However, it requires repeated infusions to keep working. Researchers led by Yoram Yovell are exploring another option: ultra-low doses of the opioid buprenorphine.
In a study published in the American Journal of Psychiatry in 2015, Yovell and colleagues compared low-dose buprenorphine to placebo in 62 patients with no history of substance abuse who had been contemplating suicide for a week or more. Many had attempted suicide before, and more than half met the criteria for borderline personality disorder.
Buprenorphine was administered under the tongue, in doses of 0.1 mg once or twice a day. The researchers used these low doses to minimize the side effects of a drug that could potentially be addictive. Those randomized to receive buprenorphine saw greater reductions in suicidal ideation compared to those who received placebo, both after two weeks and after four weeks.
Use of antidepressants did not affect the likelihood that patients would respond to buprenorphine. The researchers suggest that buprenorphine specifically treats suicidal thoughts, rather than improving depression in general.
Patients with borderline personality disorder, who are often unresponsive to medication, also saw improvement in suicidal ideation after taking buprenorphine, suggesting that the opioid treated a particular symptom of their disorder—sensitivity to feelings of separation from the people with whom they are close.
Patients did not experience withdrawal when they discontinued buprenorphine. Side effects included fatigue, nausea, dry mouth, and constipation. Patients who started out taking 0.2 mg per day were much more likely to drop out than those who started at 0.1 mg per day.
There is another reason the researchers used very low doses. A potential benefit to ultra-low–dose buprenorphine is that even a week’s supply of the drug would not produce a dangerous overdose, so patients could potentially be prescribed a week’s worth of medication to take at home instead of in an inpatient setting.
Buprenorphine is not recommended for patients with a history of substance abuse. The study only explored short-term use of the drug, and replication studies are needed to clarify its effects.
Laughing Gas Reduces Depression for Up to 24 Hours
In a proof-of-concept study presented at the 2015 meeting of the Society of Biological Psychiatry, Charles R. Conway and Peter Nagele showed that an hour of 50% oxygen/50% nitrous oxide reduced depression more than placebo as measured 2 hours and 24 hours later. Twenty patients were randomized to receive the laughing gas combination or a placebo combination made up of 50% oxygen/50% nitrogen. In the laughing gas group, four patients responded to the treatment and three patients achieved remission, compared to only one patient responding in the placebo group.
Like the anesthetic ketamine, which can bring about rapid but temporary antidepressant effects when delivered intravenously, nitrous oxide is an NMDA receptor antagonist.
Genetic Variation Predicts Which Type of Antidepressant Will Be Effective
 In a six-month study of Caucasian patients, normal variations in the gene that is responsible for brain-derived neurotrophic factor (BDNF) predicted whether patients would respond better to a selective serotonin reuptake inhibitor (SSRI) antidepressant versus a serotonin and norepinephrine reuptake inhibitor (SNRI) or a tricycle antidepressant. There are several common variants of the BDNF gene, depending on which types of amino acids appear in its coding—valine or methionine. Patients with the most common version, two valines (or Val66Val), responded better to SSRIs. About two-thirds of the population has this version of the gene, which functions most efficiently. The remaining third have at least one methionine in the BDNF gene. Patients with a Met variation responded better to SNRIs and tricyclic antidepressants.
In a six-month study of Caucasian patients, normal variations in the gene that is responsible for brain-derived neurotrophic factor (BDNF) predicted whether patients would respond better to a selective serotonin reuptake inhibitor (SSRI) antidepressant versus a serotonin and norepinephrine reuptake inhibitor (SNRI) or a tricycle antidepressant. There are several common variants of the BDNF gene, depending on which types of amino acids appear in its coding—valine or methionine. Patients with the most common version, two valines (or Val66Val), responded better to SSRIs. About two-thirds of the population has this version of the gene, which functions most efficiently. The remaining third have at least one methionine in the BDNF gene. Patients with a Met variation responded better to SNRIs and tricyclic antidepressants.
The study by R. Colle and colleagues was published in the Journal of Affective Disorders in 2015. Of the patients who were prescribed SSRIs, 68.1% of patients with the Val/Val version responded to the medication after three months, compared to 44% of the patients with a Met version. Of patients prescribed SNRIs or tricyclics, 60.9% of the Met patients reached remission by six months, compared to only 33.3% of the Val/Val patients.
Editor’s Note: In an earlier BNN we reported that according to research published by Gonzalo Laje and colleagues in the journal Biological Psychiatry in 2012, depressed patients with the better functioning Val66Val allele of BDNF respond best to ketamine, while those with the intermediate functioning Val66Met allele respond less well.
Rapid-Onset Antidepressant Treatments
At the International College of Neuropsychopharmacology (CINP) World Congress of Neuropsychopharmacology in 2014, several presentations and posters discussed treatments that bring about rapid-onset antidepressant effects, including ketamine, isoflurane, sleep deprivation, and scopolamine.
Ketamine’s Effects
Multiple studies, now including more than 23 according to researcher William “Biff” Bunney, continue to show the rapid-onset antidepressant efficacy of intravenous ketamine, usually at doses of 0.5 mg/kg over 40 minutes. Response rates are usually in the range of 50–70%, and effects are seen within two hours and last several days to one week. Even more remarkable are the six studies (two double-blind) reporting rapid onset of antisuicidal effects, often within 40 minutes and lasting a week or more. These have used the same doses or lower doses of 0.1 to 0.2mg/kg over a shorter time period.
Attempts to sustain the initial antidepressant effects include repeated ketamine infusions every other day up to a total of six infusions, a regimen in which typically there is no loss of effectiveness. Researcher Ronald Duman is running a trial of co-treatment with ketamine and lithium, since both drugs block the effects of GSK-3, a kinase enzyme that regulates an array of cellular functions, and in animals the two drugs show additive antidepressant effects. In addition, lithium has been shown to extend the acute antidepressant effects of one night of sleep deprivation, which are otherwise reversed by a night of recovery sleep.
Ketamine’s effects are related to the neurotransmitter glutamate, for which there are several types of receptors, including NMDA and AMPA. Ketamine causes a large burst of glutamate presumably because it blocks NMDA glutamate receptors on inhibitory interneurons that use the neurotransmitter GABA, causing glutamatergic cells to lose their inhibitory input and fire faster. While ketamine blocks the effects of this glutamate release at NMDA receptors, actions at AMPA receptors are not blocked, and AMPA activity actually increases. This increases brain-derived neurotrophic factor (BDNF), which is also required for the antidepressant effects of ketamine. Ketamine also increases the effects of mTOR, a kinase enzyme that regulates cell growth and survival, and if these are blocked with the antibiotic rapamycin, antidepressant effects do not occur.
In animal studies, ketamine increases dendritic spine growth and rapidly reverses the effects of chronic mild unpredictable stressors on the spines (restoring their mature mushroom shape and increasing their numbers), effects that occur within two hours in association with its rapid effects on behaviors that resemble human depression.
About 50–70% of treatment-resistant depressed patients respond to ketamine. However, about one-third of the population has a common genetic variation of BDNF in which one or both valine amino acids that make up the typical val-66-val allele are replaced with methionine (producing val-66-met proBDNF or met-66-met proBDNF). The methionine variations result in the BDNF being transported less easily within the cell. Patients with these poorly functioning alleles of BDNF are less likely to get good antidepressant effects from treatment with ketamine.
Ketamine in Animal Studies
Researcher Pierre Blier reviewed the effects of ketamine on the neurotransmitters serotonin, norepinephrine, and dopamine. In rodents, a swim stress test is used to measure depression-like behavior. Researchers record how quickly the rodents give up trying to get out of water and begin to float instead. Blier found that ketamine’s effects on swim stress were dependent on all three neurotransmitters. For dopamine, ketamine’s effects were dependent on increases in the number of dopamine cells firing, not on the firing rate, and for norepinephrine, ketamine’s effects were dependent on increases in burst firing patterns. Each of these effects was dependent on glutamate activity at AMPA receptors. Given these effects, Blier believes that using ketamine as an adjunct to conventional antidepressants that tend to increase these neurotransmitters may add to its clinical effectiveness.
Important Anecdotal Clinical Notes
Blier reported having given about 300 ketamine infusions to 25 patients, finding that two-thirds of these patients responded, including one-third who recovered completely, while one-third did not respond to the treatment. Patients received an average of 12 infusions, not on a set schedule, but according to when they began to lose response to the last ketamine infusion. If a patient had only a partial response, Blier gave the next ketamine treatment at a faster rate of infusion and was able to achieve a better response. These clinical observations are among the first to show that more than six ketamine infusions may be effective and well tolerated. Read more
The Good and Bad News About Deep Brain Stimulation for Treatment-Resistant Depression
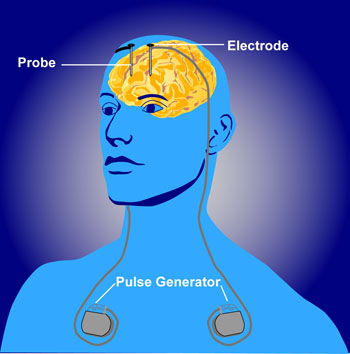 Deep brain stimulation is a treatment in which electrodes are implanted in the brain to treat movement or affective disorders. At the 2014 meeting of the International College of Neuropsychopharmacology, Thomas Schlaepfer reviewed the current status of studies of deep brain stimulation for depression. The bad news is that two double-blind randomized controlled studies are no longer recruiting patients because interim analysis failed to show a benefit to the deep brain stimulation over a sham stimulation. The studies targeted two of the most promising parts of the brain for deep brain stimulation—the subgenual anterior cingulate (important for motivation) and the anterior limb of the internal capsule (which contains nerve fibers going to and from the cerebral cortex), so their failure is a disappointment. However, Helen Mayberg, one of the lead researchers studying the subgenual anterior cingulate, will continue to study this target for deep brain stimulation.
Deep brain stimulation is a treatment in which electrodes are implanted in the brain to treat movement or affective disorders. At the 2014 meeting of the International College of Neuropsychopharmacology, Thomas Schlaepfer reviewed the current status of studies of deep brain stimulation for depression. The bad news is that two double-blind randomized controlled studies are no longer recruiting patients because interim analysis failed to show a benefit to the deep brain stimulation over a sham stimulation. The studies targeted two of the most promising parts of the brain for deep brain stimulation—the subgenual anterior cingulate (important for motivation) and the anterior limb of the internal capsule (which contains nerve fibers going to and from the cerebral cortex), so their failure is a disappointment. However, Helen Mayberg, one of the lead researchers studying the subgenual anterior cingulate, will continue to study this target for deep brain stimulation.
The better news is that Schlaepfer repositioned the electrodes to target a site in the medial forebrain bundle nearer to the ventral tegmental area. After this shift he observed rapid onset of antidepressant response (within two days) in seven of the first eight patients studied, and these responses persisted over many months of follow up. This response was achieved at 2.8 microamps, a lower stimulation current than was used in other studies of deep brain stimulation.
Editor’s Note: Since patients started to feel better when they were still on the operating table, this may offer an opportunity to more rapidly assess effectiveness, do a double-blind study, and see if the findings can be replicated as another mode of achieving rapid-acting and long-lasting antidepressant effects in treatment-resistant patients. Intravenous ketamine has rapid-onset antidepressant effects, but its effects are short-lived.
A Paradigm for Treatment of Severe PTSD developed by Dr. David Bakish
In an earlier BNN we mistakenly attributed the protocol developed by David Bakish, a renowned Canadian psychopharmacologist, to another doctor named Vaishali P. Bakshi. Our apologies to both individuals.
Dr. David Bakish is Medical Director at the Ottawa Psychopharmacology Clinic and a Former Professor of Psychiatry at the University of Ottawa in Ottawa, Ontario. He shared with this editor his novel treatment strategy for patients with exceptionally profound degrees of post-traumatic stress disorder (PTSD), which, particularly among military veterans, can be compounded by traumatic brain injury. He has had a distinguished academic career with an extensive CV and credentials including membership in the International College of Neuropsychopharmacology (CINP), the Royal College of Physicians and Surgeons of Canada, and the Canadian and European Colleges of Neuropsychopharmacology. Most importantly he has had great success in treating large numbers of patients with severe PTSD. Treatment options based on placebo-controlled clinical trials are sometimes insufficient for the treatment of seriously ill patients. FDA-approved treatment for PTSD consists of serotonin-selective antidepressants, while exposure therapies (in which the patient is gradually exposed to more of the stimuli that triggered symptoms) are the recommended psychotherapy, but these methods often leave patients highly disabled. We relay Dr. Bakish’s treatment strategy with several caveats.
Most of Bakish’s suggestions are “off-label” treatments for the treatment of PTSD or traumatic brain injury, i.e. treatments that are not FDA-approved for these purposes. In some of these instances, there is no controlled research to support the use of these drugs in patients with PTSD. Thus the ideas noted here are anecdotal, based on his personal experience, and have not been tested in controlled clinical trials. Accordingly, patients with their physicians must make their own decisions about any of the strategies reported in this or other issues of the BNN.
Bakish’s typical treatment algorithm goes well beyond the usual treatment guidelines to find solutions for hard-to-treat patients. Bakish first addresses sleep disturbance, which is almost universal in PTSD. He suggests the anticonvulsant levetiracetam (Keppra), for the hyperarousal and sleep disorder. He uses starting at doses of 125mg per night and increases by 125mg every three weeks. Read more
How Inflammation Increases Glutamate Overexcitation And Neurotoxicity
Research has shown a link between inflammation and mental illness. Inflammation leads to a series of chemical changes that can overexcite neurons and interfere with the protection of neurons.
Inflammation increases the production of indoleamine-pyrrole 2,3-dioxygenase (IDO), an enzyme that breaks down the amino acid tryptophan into kynurenic acid and quinolinic acid. They in turn increase glutamate, the main excitatory neurotransmitter, and decrease brain-derived neurotrophic factor (BDNF), which keeps neurons healthy.
Kynurenic acid stimulates microglia, which clean up the central nervous system as a form of immune defense, to produce inflammatory cytokine proteins.
Quinolinic acid directly stimulates glutamate receptors and encourages glutamate release from astrocytes. Quinolinic acid also blocks glutamate removal that would normally occur through reuptake into the astrocytes, leading to more stimulation of extrasynaptic glutamate receptors and decreases in BDNF.
Quinolinic acid’s effects are opposite to those of the antidepressant ketamine, which blocks glutamate NMDA receptors and increases BDNF. When people are given interferon protein for the treatment of cancers, quinolinic acid increases in cerebrospinal fluid, inducing depression. The severity of depression induced is correlated with the patient’s levels of quinolinic acid.
It appears that ketamine has indirect anti-inflammatory effects through its ability to block glutamate receptors and increase BDNF.
The Nucleus Accumbens in Depression
Brain-derived neurotrophic factor (BDNF) keeps neurons healthy and is critical for long-term memory and synapse formation. BDNF levels increase in the nucleus accumbens (the brain’s reward center) and decrease in the hippocampus during clinical depression and chronic cocaine use. In rodents, the same changes in BDNF levels occur during defeat stress (which resembles human depression).
Rodents who are repeatedly defeated by a larger rodent exhibit behaviors such as social withdrawal, lethargy, and decreased interest in sucrose. The increases in BDNF in the nucleus accumbens of these rodents could reflect the learning that takes place during the repeated defeat stress and the depression-like behaviors that follow it. Blocking the BDNF increases in the nucleus accumbens prevents these behaviors from developing.
Chadi Abdallah and other researchers at Yale University recently found that the left nucleus accumbens of patients with treatment-resistant depression is enlarged compared to normal controls, and the drug ketamine, which produces rapid-onset antidepressant effects, rapidly decreases the volume of the nucleus accumbens in the depressed patients. The mechanism by which it does so is unknown, but could reflect some suppression of the depressive learning.
Any relationship between the volume of the nucleus accumbens and its levels of BDNF is unknown, but ketamine’s effect on the size of this brain region could be linked to a decrease in the defeat-stress memories.
Another Blocker of Glutamate Receptor Function with Rapid Antidepressant Effects
Certain drugs such as ketamine and memantine that work by blocking activity at the NMDA receptor for the excitatory neurotransmitter glutamate have antidepressant effects. D-cycloserine is a drug that has a related mechanism and is being studied as an antidepressant. At high doses the drug acts as an antagonist at the glycine site of the NMDA receptor, blocking glycine’s ability to facilitate glutamate transmission through the receptor.
Joshua Kantrowitz, a researcher at Columbia University, reported at a recent scientific meeting that the rapid-onset antidepressant effects of D-cycloserine could be maintained for eight weeks. Similar findings were published in the Archives of General Psychiatry in 2010 and were reported in another study by Uriel Heresco-Levy in a 2013 article in the Journal of Neuropsychopharmacology.
Glutamate is the major excitatory neurotransmitter in the brain and is important for the development of long-term memory. However, glutamate overactivity may contribute to depression. Decreasing this overactivity (with ketamine, memantine, or D-cycloserine) may produce antidepressant effects.