TDCS Better Than Placebo But Not as Good as Escitalopram at Improving Unipolar Depression
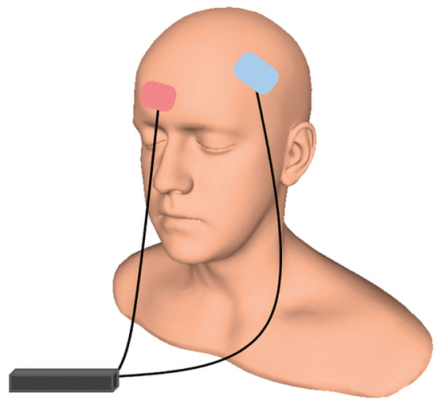 An article by André R. Brunoni and colleagues in the New England Journal of Medicine reports that transcranial direct current stimulation (tDCS) can treat unipolar depression more effectively than placebo, but not quite as effectively as the selective serotonin reuptake inhibitor (SSRI) antidepressant escitalopram. TDCS consists of a constant, low direct current applied to the scalp via electrodes.
An article by André R. Brunoni and colleagues in the New England Journal of Medicine reports that transcranial direct current stimulation (tDCS) can treat unipolar depression more effectively than placebo, but not quite as effectively as the selective serotonin reuptake inhibitor (SSRI) antidepressant escitalopram. TDCS consists of a constant, low direct current applied to the scalp via electrodes.
The study included 245 patients with moderate to severe depressive symptoms, many of whom also had anxiety disorders. To minimize the placebo effect, patients all participated in sessions wearing tDCS gear and received a daily pill. However, one group received real tDCS treatment but placebo pills, a second group received sham tDCS but real escitalopram pills, and the third group received both sham tDCS and placebo pills.
The real tDCS treatment consisted of 30-minute sessions of tDCS every day for 15 consecutive weekdays, then seven once-a-week treatments. The escitalopram dosage was 10 mg/day for three weeks, then 20 mg daily.
Ten weeks into treatment, those who received escitalopram showed the greatest improvement in depression. Those who received tDCS showed slightly less improvement, but still significantly more than those who received neither treatment. Cognitive performance either improved or stayed the same in all the groups.
In terms of side effects, those who received escitalopram were more likely to report sleepiness or severe constipation. Those who received tDCS reported more skin redness/tingling, itching, tinnitus, and nervousness. Two patients in the tDCS group had new-onset mania during treatment. There were no suicides, hospitalizations, or other serious side effects.
Taking SSRI Antidepressants May Increase Stroke Risk
 A Taiwanese study published in the Journal of Clinical Psychiatry in 2017 finds that taking selective serotonin reuptake inhibitor (SSRI) antidepressants can increase risk of stroke. The study by Chin-Hong Chan and colleagues analyzed eight years of data from Taiwan’s National Health Insurance Research Database, comparing people who had taken SSRIs for at least two consecutive months to those who had not. First onset strokes were more common among people who had taken SSRIs, and the higher stroke rates in this group persisted for three years after exposure.
A Taiwanese study published in the Journal of Clinical Psychiatry in 2017 finds that taking selective serotonin reuptake inhibitor (SSRI) antidepressants can increase risk of stroke. The study by Chin-Hong Chan and colleagues analyzed eight years of data from Taiwan’s National Health Insurance Research Database, comparing people who had taken SSRIs for at least two consecutive months to those who had not. First onset strokes were more common among people who had taken SSRIs, and the higher stroke rates in this group persisted for three years after exposure.
Ischemic strokes (which occur when a blood vessel carrying blood to the brain is obstructed) were more common than hemorrhagic strokes (which occur when a weak blood vessel ruptures). Younger adult participants exposed to SSRIs were more likely to have strokes, while people older than 65 saw only a slight increase in stroke risk from taking SSRIs. More strokes occurred during the first three years of SSRI treatment than later in treatment.
Chan and colleagues suggest that these strokes are caused by cerebral microbleeding or by overcorrection of hemostasis, the process by which the body slows or stops bleeding by constricting blood vessels and coagulating blood.
Best Antidepressants for Post-Stroke Depression
 A recent meta-analysis in the journal BMJ Open analyzes the efficacy and tolerability of 10 different antidepressants given to treat depression following a stroke. The meta-analysis incorporated data from 12 trials and a total of 707 participants. Reboxetine was the most effective antidepressant, followed by paroxetine, doxepin, and duloxetine. Sertraline, fluoxetine, and nefiracetam failed to outperform placebo in the treatment of post-stroke depression.
A recent meta-analysis in the journal BMJ Open analyzes the efficacy and tolerability of 10 different antidepressants given to treat depression following a stroke. The meta-analysis incorporated data from 12 trials and a total of 707 participants. Reboxetine was the most effective antidepressant, followed by paroxetine, doxepin, and duloxetine. Sertraline, fluoxetine, and nefiracetam failed to outperform placebo in the treatment of post-stroke depression.
In terms of tolerability, paroxetine had the least side effects and led to significantly fewer discontinuations than doxepin, citalopram, and fluoxetine. After paroxetine, the most tolerable drugs were sertraline and nortriptyline. The least tolerable drug was citalopram.
Researchers led by Yefei Sun suggested that paroxetine might be the best antidepressant to prescribe after a stroke due to its efficacy and good tolerability. Fluoxetine might be the worst due to its poor efficacy and poor side effects profile.
Editor’s Note: Multiple randomized controlled trials suggest that antidepressants can be helpful for anyone who has a stroke, both to decrease depression and to improve neurological and functional outcomes.
Botox for Depression
 Several recent clinical trials have suggested that Botox injections between the eyebrows may improve depression. The theory is that decreasing muscle tension could reduce feelings of depression, instead of depression causing muscle tension. In a phase 2 double blind multicenter trial of 258 women with depression, participants were randomized to receive 30 units of Botox, 50 units of Botox, or placebo. Those who received the 30-unit injections showed significantly greater improvement in depression at three weeks and nine weeks compared to those who received placebo. However, it was not superior to placebo at the primary endpoint of the study, six weeks, and the 50-unit dosage was not superior to placebo. Both doses were well tolerated.
Several recent clinical trials have suggested that Botox injections between the eyebrows may improve depression. The theory is that decreasing muscle tension could reduce feelings of depression, instead of depression causing muscle tension. In a phase 2 double blind multicenter trial of 258 women with depression, participants were randomized to receive 30 units of Botox, 50 units of Botox, or placebo. Those who received the 30-unit injections showed significantly greater improvement in depression at three weeks and nine weeks compared to those who received placebo. However, it was not superior to placebo at the primary endpoint of the study, six weeks, and the 50-unit dosage was not superior to placebo. Both doses were well tolerated.
Botox is derived from botulinum toxin, which can relax tense muscles. It is also being explored as a treatment for migraine headaches. The manufacturer, Allergan, expects to move forward with phase 3 trials of Botox for depression.
Intranasal Ketamine for Bipolar Disorder
 An in-press article due out in January 2018 by Demitri F. Papolos and colleagues in the Journal of Affective Disorders reports that intranasal ketamine delivered every three to four days reduced symptoms of bipolar disorder in 45 teens (aged 16 years on average). The teens treated in one private practice had the ‘fear-of-harm’ subtype, which in addition to bipolar symptoms is characterized by treatment resistance, separation anxiety, aggressive obsessions, disordered sleep, and poor temperature regulation.
An in-press article due out in January 2018 by Demitri F. Papolos and colleagues in the Journal of Affective Disorders reports that intranasal ketamine delivered every three to four days reduced symptoms of bipolar disorder in 45 teens (aged 16 years on average). The teens treated in one private practice had the ‘fear-of-harm’ subtype, which in addition to bipolar symptoms is characterized by treatment resistance, separation anxiety, aggressive obsessions, disordered sleep, and poor temperature regulation.
The repeated administration of ketamine produced long-lasting positive results, improving bipolar symptoms as well as social function and academic performance. Many participants reported via survey that they were much or very much improved after being treated for durations ranging from 3 months to 6.5 years. Side effects were minimal and included sensory problems, urination problems, torso acne, dizziness, and wobbly gait.
The ketamine was delivered to alternating nostrils via 0.1 ml sprays that included 50–200 mg/ml of ketamine in 0.01% benzalkonium chloride. Patients were instructed to increase the dosage just up until it became intolerable and then repeat the last tolerable dose every three to four days. Final doses ranged from 20–360 mg. The mean dose was 165 mg (plus or minus 75 mg) delivered every 3 days.
Papolos and colleagues called for placebo-controlled clinical trials based on the positive results from this open study.
An Overview of Ketamine for Treatment-Resistant Depression
 A 2017 series of articles by researcher Chittaranjan Andrade in the Journal of Clinical Psychiatry reviews the last 10 years of research on ketamine, the anesthetic drug that in smaller doses (0.5 mg/kg of body weight) can bring about rapid antidepressant effects. Ketamine is typically delivered intravenously (though it can also be delivered via inhaler, injected under the skin or into muscles, and least effectively by mouth). Ketamine can improve depression in less than an hour, but its effects usually fade within 3 to 5 days. Repeating infusions every few days can extend ketamine’s efficacy for weeks or months.
A 2017 series of articles by researcher Chittaranjan Andrade in the Journal of Clinical Psychiatry reviews the last 10 years of research on ketamine, the anesthetic drug that in smaller doses (0.5 mg/kg of body weight) can bring about rapid antidepressant effects. Ketamine is typically delivered intravenously (though it can also be delivered via inhaler, injected under the skin or into muscles, and least effectively by mouth). Ketamine can improve depression in less than an hour, but its effects usually fade within 3 to 5 days. Repeating infusions every few days can extend ketamine’s efficacy for weeks or months.
Andrade cited a 2016 meta-analysis of nine ketamine studies by T. Kishimoto and colleagues in the journal Psychological Research. The meta-analysis found that compared to placebo, ketamine improved depression beginning 40 minutes after IV administration. Its effects peaked at day 1 and were gone 10–12 days later. Remission rates were better than placebo starting after 80 minutes and lasting 3–5 days.
Several studies have found that ketamine also reduces suicidality.
Andrade reported that both effectiveness and side effects seem to be dose-dependent within a range from 0.1 mg/kg to 0.75 mg/kg.
Side effects of ketamine are typically mild and transient. A 2015 study by Le-Ben Wan and colleagues (also in the Journal of Clinical Psychiatry) that Andrade cited reported that in 205 sessions of ketamine administration, the most common side effects were drowsiness, dizziness, poor coordination, blurred vision, and feelings of strangeness or unreality. The feelings of unreality (dissociative effects) diminish with repeated infusions. Heart and blood pressure may also temporarily increase as a result of ketamine administration.
One study found that ketamine could speed up and add to the effects of the selective serotonin reuptake inhibitor (SSRI) antidepressant escitalopram (Lexapro). A meta-analysis of 10 randomized controlled trials found that ketamine did not improve the effects of electroconvulsive therapy.
Ketamine has some history as a recreational club drug (sometimes known as ‘K’ or ‘special K’), and can be misused or abused.
While there have been many studies of ketamine’s antidepressant effects, Andrade concludes that none is of a standard to justify US Food and Drug Administration approval for the drug. It is hoped that larger, more rigorous trials will be completed in the next few years. However, ketamine is already being used widely to treat treatment-resistant unipolar and bipolar depression.
Vagus Nerve Stimulation Improves Depression When Other Treatments Fail
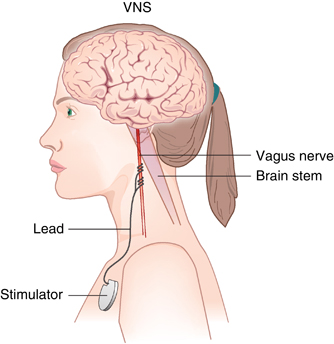 Vagus nerve stimulation (VNS) has been approved by the US Food and Drug Administration as an adjunctive therapy for treatment-resistant unipolar and bipolar depression since 2005. The treatment consists of a pacemaker-like device implanted under the skin in the chest that delivers regular, mild electrical pulses to the brain via the left vagus nerve.
Vagus nerve stimulation (VNS) has been approved by the US Food and Drug Administration as an adjunctive therapy for treatment-resistant unipolar and bipolar depression since 2005. The treatment consists of a pacemaker-like device implanted under the skin in the chest that delivers regular, mild electrical pulses to the brain via the left vagus nerve.
A 2017 study by Scott T. Aaronson and colleagues in the American Journal of Psychiatry reports that over a 5-year period, people with treatment-resistant depression who received VNS did better than those who received treatment as usual. The 795 participants at 61 US sites had either a depressive episode that had lasted for at least two years or had had three or more depressive episodes and had failed to respond to at least four treatments, including electroconvulsive therapy (ECT). Over five years, those who received VNS had higher response rates (67.6% versus 40.9%) and higher remission rates (43.3% versus 25.7%) compared to those who received treatment as usual.
While the study by Aaronson and colleagues was non-blind and non-randomized, it suggests that VNS could be helpful in the long-term management of treatment-resistant unipolar and bipolar depression.
Editor’s Note: VNS was FDA-approved for treatment-resistant seizures in patients aged 12 and older in 1997 and for children 4 years and older in 2017. It was also approved for cluster headaches in 2017. Insurance coverage and reimbursement for VNS is typically available for these neurological conditions, but not for the treatment of depression. This is an unfortunate example of the stigmatization of psychiatric illness—when an FDA-approved device can be kept from people in need of treatment.
One Night of Sleep Deprivation Can Rapidly Improve Depression
 One night of sleep deprivation can bring about rapid improvement in depression symptoms the following day. A 2017 meta-analysis by Elaine M. Boland and colleagues in the Journal of Clinical Psychiatry summarizes the findings from 66 studies of sleep deprivation for unipolar and bipolar depression and finds that the technique produced a response rate of 45% in randomized controlled studies.
One night of sleep deprivation can bring about rapid improvement in depression symptoms the following day. A 2017 meta-analysis by Elaine M. Boland and colleagues in the Journal of Clinical Psychiatry summarizes the findings from 66 studies of sleep deprivation for unipolar and bipolar depression and finds that the technique produced a response rate of 45% in randomized controlled studies.
It did not seem to matter whether patients experienced full or partial sleep deprivation, whether they had unipolar or bipolar depression, or whether or not they were taking medication at the time. Age and gender also did not affect the results of the sleep deprivation.
Extending the Effects
While sleep deprivation can rapidly improve depression, the patient often relapses after the next full night of sleep. There are a few things that can prevent relapse or extend the efficacy of the sleep deprivation. The first is lithium, which has extended the antidepressant effects of sleep deprivation in people with bipolar disorder.
The second strategy to prevent relapse is a phase change. This means going to sleep early in the evening the day after sleep deprivation and gradually shifting the sleep schedule back to normal. For example, after an effective night of sleep deprivation, one might go to sleep at 6pm and set their alarm for 2am. Then the next night, they would aim to sleep from 7pm to 3am, the following night 8pm to 4am, etc. until the sleep wake cycle returns to a normal schedule.
A 2002 article by P. Eichhammer and colleagues in the journal Life Sciences suggested that repetitive transcranial magnetic stimulation (rTMS), electromagnetic stimulation of the scalp over the prefrontal cortex, could help maintain improvement in depression following a night of partial sleep deprivation for up to four days.
Bright light therapy may also help. Read more
Inflammation Predicts Poor Response to Sleep Deprivation with Light Therapy
A 2017 article by Francesco Benedetti and colleagues in the Journal of Clinical Psychiatry reports that people with bipolar depression who have higher levels of certain inflammatory markers may have a poor antidepressant response to the combination of sleep deprivation and light therapy, compared to those with lower levels of inflammation.
The study included 37 participants with bipolar disorder who were in the midst of a major depressive episode. Of those, 31 participants (84%) had a history of poor response to antidepressant medication. The patients were treated with three cycles of total sleep deprivation and light therapy within one week, a combination that can often bring about a rapid improvement in depression.
Depression improved in a total of 23 patients (62%) following the therapy. Blood analysis showed that compared to those who had a good response, the non-responders had higher levels of five intercorrelated inflammatory markers: IL-8, MCP-1, IFN-gamma, IL-6, and TNF-alpha. Those with higher body mass index had more inflammation, indirectly decreasing response to the therapy.
FDA Approves Extended-Release Aripiprazole Injected Monthly to Prevent Manic and Mixed Episodes in Bipolar I
In 2017 the US Food and Drug Administration approved a monthly injectable form of the atypical antipsychotic drug aripiprazole, Abilify Maintena, for the prevention of manic and mixed episodes in bipolar I disorder. The intramuscular injections are available for monotherapy in preparations of 300 mg or 400 mg. Maintena did not prevent depressive episodes.
Maintena is already FDA-approved for the treatment of schizophrenia and Tourette’s syndrome in adults.
The approval for bipolar I disorder follows a 52-week phase 3, double-blind, placebo-controlled randomized trial. Participants were experiencing a manic episode during screening for the study, met the criteria for bipolar I disorder, and had had at least one prior manic or mixed episode severe enough to require treatment.
Compared to placebo, Maintena in once-a-month injections delayed the recurrence of any mood episode following the initial manic episode at screening. When the researchers separated their analysis based on type of episode, Maintena reduced manic and mixed episodes compared to placebo, but did not do a better job than placebo at preventing depressive episodes.
An oral antipsychotic must be administered for 14 days following the first injection of Maintena. The extended-release injection is available as 300 mg– or 400 mg–strength powder that may be reconstituted, or as prefilled syringes.
Editor’s Note: Because Maintena is delivered as a once-a-month injection, it may be helpful for patients who struggle to take daily oral medications.