Treating Bipolar Depression in an Adolescent
At the 2019 meeting of the International Society for Bipolar Disorders, researcher Ben Goldstein discussed a case of a 15-year-old with bipolar depression and his recommended treatments for the adolescent. Goldstein endorsed the use of an atypical antipsychotic such as lurasidone, and perhaps also quetiapine. Goldstein noted 2015 findings from researcher Robert Findling that lamotrigine was significantly more effective than placebo in adolescents 13–18 years old, but was not effective in those aged 10–12.
(In adults, researcher John Geddes and colleagues found that in patients with an inadequate antidepressant response to quetiapine, the addition of lamotrigine was more effective than adding a placebo, both acutely and in long-term follow-up. The only caveat was that lamotrigine was less effective in those who were also being treated with folate.)
Editor’s Note: Some other treatments could augment the effects of the regimen proposed by Goldstein, including lithium and the antioxidant N-acetylcysteine, which, it should be noted, takes more than eight weeks to become effective. Vitamin D3 could also be considered, as it is often low in children with psychiatric disorders. One treatment that went unmentioned at the meeting was repeated transcranial magnetic stimulation, or rTMS, which is effective and well-tolerated in adolescents with depression.
For patients with more rapidly cycling bipolar disorder and only partial response to medications, the combination of the ‘three Ls’ (lurasidone, lamotrigine, and lithium) could have considerable appeal, given that each drug is from a different class of medications, has a different mechanism of action, targets a different mood phase, and is relatively well-tolerated both alone and in combination with other drugs.
Antioxidant Supplement Coenzyme Q10 Looks Promising for Bipolar Depression
Coenzyme Q10 (CoQ10) is an antioxidant that occurs naturally in the human body, but its levels decline with age, medical illness, and depression. In a randomized, controlled trial that was published in the Journal of Clinical Psychopharmacology in 2018, researcher Maryam Mehrpooya and colleagues found that adding coenzyme Q10 supplements to a treatment regimen improved bipolar depression compared to adding placebo.
The pathophysiology of bipolar disorder involves mitochondrial dysfunction, oxidative stress, and inflammation, and coenzyme Q10 can affect all of these pathways. It is also neuroprotective, and may help prevent the degeneration of neurons in people with Alzheimer’s, Parkinson’s, or Huntington’s diseases.
The study included a final total of 69 participants who were randomly assigned to receive either 200 mg/day of coenzyme Q10 supplements or placebo in addition to their normal treatment regimen, which had been stable for at least two months at the time of the study. Participants’ bipolar depression was rated at the beginning of the study, after four weeks, and after eight weeks. At the eight-week mark, coenzyme Q10 showed a statistically significant benefit over placebo with a large effect size. Three participants who received coenzyme Q10 experienced full remission of their depression, and 72% of those in the coenzyme Q10 group improved compared to only 12% of those who received placebo.
The study had some limitations. It was small, and twenty participants dropped out of the study before its completion, which may have inflated the findings.
Previous research found that coenzyme Q10 had benefits in specific populations. In two non-blind studies (studies in which participants know that they are receiving the treatment in question rather than possibly a placebo), 29 older patients with bipolar disorder improved when taking 800 mg to 1200 mg/day of coenzyme Q10. A randomized, controlled trial of coenzyme Q10 in people with multiple sclerosis and depression found that 500 mg/day reduced fatigue symptoms and depression. Coenzyme Q10 has also improved well-being and energy in small, controlled trials in people with breast cancer, Gulf War veterans, and elderly populations.
Taking coenzyme Q10 is low-risk. It had no adverse effects in the study by Mehrpooya and colleagues. Gastrointestinal reactions are possible, but can be managed by taking coenzyme Q10 with food and spreading out dosing throughout the day. Insomnia is also possible, but is less likely when coenzyme Q10 is taken early in the day. One effect to note is that coenzyme Q10 can interact badly with the blood-thinner warfarin.
Editor’s Note: The study by Mehrpooya and colleagues is interesting. Another antioxidant, N-acetylcysteine (NAC), also took 2 months to work in trichotillomania and bipolar depression, so patients should be warned not to expect a quick response with either coenzyme Q10 or NAC. Other potentially useful supplements include: Vitamin D3 (1500–5000 IU/day), folate or L-methylfolate, and acetyl-L-carnitine. Acetyl-L-carnitine may work more quickly, based on its presumed mechanism (increasing the production of the inhibitory metabotrophic glutamate receptor mGluR-2, which inhibits glutamate release).
High Baseline Levels Of C-Reactive Protein Predict Better Response To Lurasidone in Bipolar Depression
In a study presented at the 2017 meeting of the International Society for Affective Disorders, Charlies L. Raison and colleagues examined whether baseline levels of the inflammatory marker C-reactive protein (CRP) affected antidepressant response to the antipsychotic drug lurasidone in bipolar depression. The participants were divided into three double-blind groups: one received 20–60mg/day of lurasidone, another received 80–120 mg/day of lurasidone, and the third received placebo over a period of six weeks. The effect was dramatic—in people with CRP levels above 5 mg/L at the beginning of the study, lurasidone (at either dosage level) had a very large effect size (d=0.85), while in people with baseline CRP levels below 5 mg/L the effect size was smaller (d=0.35).
Interestingly, 118 of the participants (24.5%) had CRP levels above 5mg/L at baseline, indicating a substantial amount of inflammation was present in a quarter of the bipolar depressed patients. Higher levels of CRP at baseline were correlated with better improvement on specific items on the Montgomery–Åsberg Depression Rating Scale (MADRS): “lassitude” (or lack of energy), “apparent sadness,” “reported sadness,” and “pessimistic thoughts.” Raison and colleagues concluded: “These findings suggest that the efficacy of lurasidone in patients with bipolar depression may in part be linked to the inflammatory status of patients prior to treatment. If confirmed in prospective investigations, [the results of a wide-range CRP assay] may prove useful as a predictive biomarker that could help optimize the use of lurasidone for the treatment of patients with bipolar depression.”
Editor’s Note: In many instances, high levels of CRP predict a poor response to treatment (such as to selective serotonin reuptake inhibitor antidepressants (SSRIs) in unipolar depression), so these findings are of considerable interest. They also suggest the untested possibility that lurasidone has anti-inflammatory effects, as those with high levels of inflammation at baseline often respond better to drugs with direct anti-inflammatory effects such as celecoxib (Celebrex) or the antioxidant N-acetylcysteine (NAC).
Third Study Suggests Cariprazine Is Effective in Bipolar Depression
The atypical antipsychotic drug cariprazine (sold under the name Vraylar in the US) is currently approved by the US Food and Drug Administration for the treatment of schizophrenia and manic or mixed episodes of bipolar disorder. Based on recent successful phase 3 trials in bipolar depression, the pharmaceutical companies that produce cariprazine, Allergan and Gedeon Richter, plan to apply for a change in FDA labeling later this year to reflect the drug’s apparent ability to treat bipolar depression as well.
While many drugs can prevent or treat mania, treating bipolar depression has typically been more of a challenge. The most recent 6-week trial of cariprazine in 493 patients showed that a dose 1.5mg/day was significantly more effective than placebo at reducing depression ratings. (A dose of 3mg/day did not show superiority over placebo as it had in previous trials of cariprazine.)
Side effects reported in the trial were mild and included restless legs, nausea, and fatigue. Five percent of those who received cariprazine discontinued the drug due to side effects, compared to three percent of those who received placebo.
The mechanism by which cariprazine improves depression is not yet clear. The drug is a dopamine partial agonist, but unlike aripiprazole (Abilify) and brexpiprazole (Rexulti), which have more potent effects on D2 receptors than on D3 receptors, cariprazine is more potent at dopamine D3 receptors. Whether this difference accounts for the positive effects in bipolar depression that aripiprazole and brexpiprazole do not have remains to be seen.
FDA Approves Lurasidone for Bipolar Depression in Children and Adolescents
 In March 2018, the US Food and Drug Administration approved the antipsychotic drug lurasidone (Latuda) for the treatment of bipolar depression in children and adolescents aged 10–17 years. Lurasidone was already approved for adults with bipolar depression, as an add-on treatment to the mood stabilizers lithium and valproate, and for schizophrenia in people aged 13 years and up.
In March 2018, the US Food and Drug Administration approved the antipsychotic drug lurasidone (Latuda) for the treatment of bipolar depression in children and adolescents aged 10–17 years. Lurasidone was already approved for adults with bipolar depression, as an add-on treatment to the mood stabilizers lithium and valproate, and for schizophrenia in people aged 13 years and up.
A 6-week clinical trial in 347 youth compared lurasidone (in doses ranging from 20 to 80 mg/day) to placebo and found that those who received lurasidone showed significant improvements in depression compared to those who received placebo. The average dose was below 40 mg/day. The research by Melissa P. DelBello and colleagues was published in the Journal of the American Academy of Child and Adolescent Psychiatry in 2017.
In the study, lurasidone was well-tolerated. Side effects included nausea, sleepiness, minimal weight gain, and insomnia. Lurasidone did not seem to affect glucose, triglycerides, cholesterol, or blood pressure.
Editor’s Note: This is the first drug to be approved for bipolar depression in this age range. This editor (Robert M. Post) has written extensively on the high incidence of childhood onset bipolar disorder in the US, and especially in the offspring of parents with bipolar disorder.
It is important to be alert to the possibilities of depression and bipolar disorder in children in the US (along with related illnesses such as anxiety, oppositional defiant disorder, and attention deficit hyperactivity disorder (ADHD)), as early-onset illness tends to have a more severe long-term course than adult-onset depression and bipolar disorder. A longer delay between the emergence of symptoms and the first treatment for bipolar disorder is also a risk factor for more severe depression, more time depressed, and a poorer outcome in adulthood.
Parents of children aged 2-12 who have mood or behavioral problems are encouraged to consider joining the Child Network at our website, bipolarnews.org (click on the tab for the Child Network). By participating in this research network, parents are able to make a weekly rating of the severity of their children’s symptoms of anxiety, depression, ADHD, oppositional behavior, and mania via the secure website. The ratings can then be shared with the child’s clinicians for easy visualization of the course of symptoms over time, which may help with treatment decisions.
Management of Unipolar and Bipolar Depression During Pregnancy
 At the Maryland Psychiatric Research Society’s continuing medical education conference in November, Lauren Osbourne, Assistant Director of the Women’s Mood Disorders Clinic at Johns Hopkins Hospital, gave a presentation on the management of mood and anxiety during pregnancy and lactation. She had a number of important ideas for physicians and patients to consider in their decision-making process.
At the Maryland Psychiatric Research Society’s continuing medical education conference in November, Lauren Osbourne, Assistant Director of the Women’s Mood Disorders Clinic at Johns Hopkins Hospital, gave a presentation on the management of mood and anxiety during pregnancy and lactation. She had a number of important ideas for physicians and patients to consider in their decision-making process.
According to Osbourne, 60%-70% of pregnant women with unipolar depression who discontinue their antidepressants relapse. Of those with bipolar disorder who discontinue their mood stabilizers, 85% relapse, while 37% of those who stay on their medications relapse.
Something to consider when deciding whether to continue medication while pregnant is that depression in pregnancy carries its own risks for the fetus. These include preterm delivery, low birth weight, poor muscle tone, hypoactivity, increased cortisol, poor reflexes, and increased incidence of attention deficit hyperactivity disorder (ADHD) and other behavioral disorders.
The placenta makes an enzyme 11-BHSD2 that lowers the stress hormone cortisol in the baby. However, this enzyme is less active in depression, exposing the fetus to higher levels of cortisol.
Thus, the decision about whether to continue medications during pregnancy should consider the risks to the fetus of both the mother’s depression and the mother’s medications.
Most antidepressants are now considered safe during pregnancy. There have been reports of potential problems, but these data are often confounded by the fact that women with more severe depression are more likely to require antidepressants, along with other risk variables such as smoking or late delivery (after 42 weeks). When these are accounted for by using matched controls, the apparent risks of certain antidepressants are no longer significant. This includes no increased risk of persistent pulmonary hypertension, autism, or cardiac malformations.
There may be a possible increased risk of Neonatal Adaption Syndrome (NAS) in the first weeks of life in babies who were exposed to selective serotonin reuptake inhibitor (SSRI) antidepressants in the third trimester. This syndrome presumably results from antidepressant withdrawal, and can include respiratory distress, temperature changes, decreased feeding, jitteriness/irritability, floppiness or rigidity, hypoglycemia, and jaundice. There is not yet a robust literature on the syndrome, but Osbourne suggested that it disappears within 2 weeks of birth.
In her practice, Osbourne prefers to prescribe sertraline, which has the best safety data, along with fluoxetine. Sertraline is also OK for breastfeeding. There is less data on bupropion, but it also appears to be safe during pregnancy. Endocrine and enzyme changes in pregnancy typically cause a 40% to 50% decrease in concentrations of antidepressants, so doses of antidepressants typically must be increased in order to maintain their effectiveness.
Osbourne ranked mood stabilizers for bipolar disorder, from safest to most worrisome. Lamotrigine is safest. There is no evidence linking it to birth defects, but higher doses are required because of increased clearance during pregnancy. Lithium is next safest. There are cardiac risks for one in 1,200 patients, but these can be monitored. Carbamazepine is third safest. One percent of babies exposed to carbamazepine will develop spina bifida or craniofacial abnormalities. Valproate is least safe during pregnancy. Seven to ten percent of babies exposed to valproate will develop neural tube defects, other malformations, or developmental delay, with a mean decrease of 9 IQ points. The atypical antipsychotics all appear safe so far.
Alternatives and Adjuncts to Medications in Pregnancy
TDCS Effective in Bipolar Depression
 A 2017 study in the journal JAMA Psychiatry reports that transcranial direct current stimulation (tDCS) is an effective add-on treatment for bipolar depression. In the study by researcher Bernardo Sampaio-Junior and colleagues, 59 patients taking medication for bipolar disorder and experiencing a depressive episode were randomized to receive either 10 daily half-hour sessions of tDCS (and then one every two weeks) or an inactive sham stimulation.
A 2017 study in the journal JAMA Psychiatry reports that transcranial direct current stimulation (tDCS) is an effective add-on treatment for bipolar depression. In the study by researcher Bernardo Sampaio-Junior and colleagues, 59 patients taking medication for bipolar disorder and experiencing a depressive episode were randomized to receive either 10 daily half-hour sessions of tDCS (and then one every two weeks) or an inactive sham stimulation.
TDCS is a painless form of neurostimulation in which electrodes applied to the scalp provide a steady, low current of electricity that modulates neuron activity. Sampaio-Junior describes its low cost, portability and ease of use as some of its benefits. This is the first randomized, sham-controlled study of tDCS in bipolar disorder.
After six weeks of treatment, patients who received real tDCS treatment showed significantly more improvement in their depression than those who received the inactive sham stimulation. In the active group, 67.6% showed sustained response compared to 30.4% in the inactive group. TDCS was well tolerated, with skin redness at the application site the only side effect that was more common in the active group than in the sham group. Mood switching rates were similar across the two groups.
The research was completed as part of the Bipolar Depression Electrical Treatment Trial (BETTER) taking place in Brazil. The group of participants was 68% female with a mean age of 45.9 years. Sixty-one percent of participants had bipolar I disorder while the remainder had been diagnosed with bipolar II.
Midday Bright Light Therapy Improved Bipolar Depression
A study by Dorothy K. Sit and colleagues published in the American Journal of Psychiatry in 2017 found that delivering bright white light therapy to patients with bipolar depression between the hours of noon and 2:30pm improved their depression compared to delivering inactive dim light, and did not cause mood switches into mania. The study included 46 patients with moderate bipolar depression, no hypomania and no psychosis.
The active therapy group was exposed to broad-spectrum bright white fluorescent light at 7,000 Lux while the inactive group received dim red light at 50 Lux. Both groups were instructed to sit 12 inches from the light and face it without looking directly at it. The therapy began with 15-minute afternoon sessions and increased to 60 minutes per day by 4 weeks. Participants were assessed weekly. Remission rates increased dramatically in the active group beginning in the fourth week. At weeks 4 through 6, the remission rate for those in the active bright light group was 68.2% compared to only 22.2% in the dim light group.
Mean depression scores were better in the treated group, as were global functioning and response rates.
Some participants were taking antidepressants concurrently, and these participants were evenly distributed across the two study groups.
An earlier pilot study by the same researchers had found that bright light therapy delivered in the morning was followed by some hypomanic reactions or bipolar cycling. The midday sessions did not cause any mood switching.
Bright light therapy is often used to treat seasonal affective disorder (SAD) using a 10,000 Lux light box. This study took place mostly during the fall and winter months.
Editor’s Note: Bright light therapy is generally safe and boasts a high remission rate. Light boxes can be acquired without a prescription and are portable and easy to use. Midday light may have the best results and the least risk of provoking a mood switch into mania.
Deep TMS May Improve Treatment-Resistant Bipolar Depression
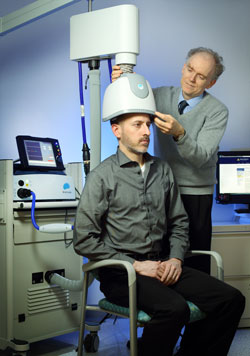 Deep transcranial magnetic stimulation (dTMS) is a non-invasive treatment that has been shown to be effective in unipolar depression. It consists of a helmet fitted to the head, which uses magnetic coils to create an electric field in a desired brain region.
Deep transcranial magnetic stimulation (dTMS) is a non-invasive treatment that has been shown to be effective in unipolar depression. It consists of a helmet fitted to the head, which uses magnetic coils to create an electric field in a desired brain region.
A 2017 double-blind randomized study by Diego F. Taveres and colleagues in the journal Neuropsychopharmacology found that 20 sessions of dTMS targeting the left dorsolateral prefrontal cortex produced greater improvement in bipolar depression over 4 weeks of treatment than the same number of sham sessions in which participants wore a helmet that delivered similar sounds and scalp sensations without the electrical effects to the brain. The participants had treatment-resistant bipolar depression that was being treated with medication.
However, dTMS’ effects were not significantly different from those of the sham over four additional weeks of follow-up, nor were remission rates significantly different across the two groups. Out of 50 participants, seven dropped out of the study—two from the sham group, and five from the active dTMS group. But there were no occasions on which a participant switched into mania following treatment.
This study suggests that dTMS has the potential to more rapidly improve treatment-resistant bipolar depression as well as unipolar depression.
Arthritis Drug Celecoxib May Improve Bipolar Depression When Paired with Escitalopram
A new study suggests that for people with bipolar depression, the anti-inflammatory drug celecoxib (Celebrex), typically used to treat arthritis, can boost the effectiveness of the antidepressant escitalopram (Lexapro).
In the 8-week study by researcher Angelos Halaris and colleagues, adults with bipolar depression were randomly assigned to one of two groups. The first group received the selective-serotonin reuptake inhibitor (SSRI) antidepressant escitalopram plus celecoxib to target inflammation. The second group received just the antidepressant escitalopram and a placebo.
By the end of the study, 78% of the group taking the anti-arthritis drug had seen major improvement in their depression, with 63% reporting that it had lifted completely. Meanwhile in the placebo group, only 45% reported major improvement, and 10% reported remission.
The group that received celecoxib with their escitalopram also began seeing improvement within one week of beginning treatment, instead of after four to six weeks, which is typical of antidepressant treatment.
Researchers think depression creates an immune response leading to chronic inflammation, which can upset the balance of neurotransmitters in the brain and make antidepressants less effective. Halaris suggests that reducing this inflammation with a drug like celecoxib can make antidepressants more effective.
The research was presented at the Fifth International Congress on Psychiatry and the Neurosciences and has not yet been published.