Lithium May Work by Restoring Dendritic Spines
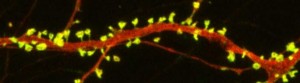
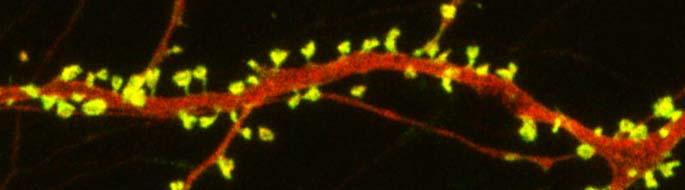
New research on mice clarifies lithium’s effects on neurons and suggests how it can lead to improved symptoms. Dendrites are the long projections on neurons that seem to reach out to form synapses with other neurons. The surface of these dendrites is covered in mushroom-shaped spines that help create these synaptic connections. A 2016 article by research Ben Cheyette and colleagues in the journal Molecular Psychiatry reports that in mice with a genetic mutation common to people with autism, schizophrenia, and bipolar disorder, lithium restored healthy numbers of the mushroom-shaped spines. The lithium treatment also reversed symptoms such as lack of interest in social interactions, lack of motivation, and anxiety in the mice.
Cheyette and colleagues first identified a genetic mutation that affects signaling in what is known as the brain’s Wnt pathway. The mutation, while rare, is 80% more common in people with bipolar disorder, autism, and schizophrenia than in people without these disorders.
When the mice were given a similar mutation, they exhibited symptoms such as anxiety, decreased sociability, and lack of motivation. They also had reduced numbers of dendritic spines and impaired Wnt signaling.
Lithium can improve Wnt signaling by blocking an enzyme called GSK-3 beta that impairs the signaling.
Treating the mice with lithium restored their dendritic spines and improved their behavior.
Wnt signaling and dendritic spines may offer the key to lithium’s success in treating a variety of psychiatric disorders in people.
Agomelatine in an Animal Model of PTSD
At the 2014 meeting of the International College of Neuropsychopharmacology, researcher Joseph Zohar presented a poster on the effects of early post-stressor intervention with the drug agomelatine in animals who showed behavioral and molecular responses to stress that served as a model of post-traumatic stress disorder (PTSD).
Agomelatine is available clinically as an antidepressant in Canada and Europe (but not in the US), and can also reduce anxiety and re-synchronize circadian rhythms. Agomelatine is a melatonin (MT1/MT2) receptor agonist and a serotonin 5HT2C antagonist (increasing dopamine and norepinephrine in the frontal cortex).
Long-term behavioral, molecular and structural effects of the drug were assessed in animals. Adult male Sprague-Dawley rats were exposed to the scent of a predator for 10 minutes, and one hour later they were treated acutely for this stress with agomelatine (50mg/kg i.p.) or placebo.
Agomelatine decreased the prevalence of extreme, PTSD-like behavioral and molecular responses to the stressor, such as freezing in place and increased corticosterone. Agomelatine also normalized decreases in brain-derived neurotrophic factor (BDNF) observed in the dentate gyrus of the hippocampus, the cortex (layer III), and the basolateral amygdala. In line with this, agomelatine-treated stressed animals displayed significantly increased number and length of dendrites at glutamate synapses in the hippocampus (including the dentate gyrus and CA1) and reversed the hippocampal neuronal retraction observed in the rats who were given the placebo.
Agomelatine also affected the expression of clock genes in the rats, which regulate biorhythms. These genes lead to the production of the major clock gene proteins Per1 and Per2. Agomelatine normalized Per1 increases in three parts of the brain: the CA3, another glutamate synapse near the dentate gyrus; the suprachiasmatic nucleus over the optic chiasm, important for circadian rhythms; and the basolateral amygdala. Per2, a protein that also drives circadian rhythms, increased in the CA1 synapse of the hippocampus, the suprachiasmatic nucleus and the basolateral amygdala of the stressed rats.
The researchers concluded that the data provide “initial evidence that a single dose of agomelatine administered in the acute aftermath of stress promotes recovery while promoting enhanced neuronal and synaptic plasticity and connectivity in the secondary prevention of PTSD in this model.”
In Mice, Autism-Like Behavior Connected to Problems Pruning Dendritic Spines
Autism spectrum disorders are associated with developmental abnormalities at excitatory synapses. Dendrites, the branched projections of neurons where electrical signals are passed from one cell to the next, are covered in hundreds to thousands of spines that facilitate the synaptic connections with other neurons. These spines are created and also pruned as part of normal learning and development.
Post-mortem examination of the brains of patients with autism spectrum disorders shows increased density of dendritic spines and less pruning in certain neurons in the temporal lobe. These examinations also show impaired mTOR autophagy. MTOR is a protein that plays a role in cell growth and survival. Autophagy is the normal process by which some components of cells are broken down.
A 2014 study by Guomei Tang et al. in the journal Neuron showed that mice that are genetically altered to have overactive mTOR also have reduced dendritic spine pruning, blockade of autophagy, and increased autism-like behaviors. An immunosuppressant drug called rapamycin inhibits mTOR, and treating the mice with this drug corrected the problems with spine pruning and the autism-like behaviors. (This was not true for mice who had been altered to have another type of autophagy.) Normal spine formation was not affected by the restored pruning ability.
Tang et al. concluded that mTOR autophagy plays an important role in dendritic spine pruning, and that restoring neuronal autophagy can correct synaptic abnormalities and restore normative social behavior in mice with hyperactive mTOR.
Psychiatric Revolution: Changes in Behavior Are Associated with Dendritic Spine Shape and Number
New research shows that cocaine, defeat stress, the rapid-acting antidepressant ketamine, and learning and memory can change the size, shape, or number of spines on the dendrites of neurons. Dendrites conduct electrical impulses into the cell body from neighboring neurons.
Cocaine
Several researchers, including Peter Kalivas at the Medical University of South Carolina, have reported that cocaine increases the size of the spines on the dendrites of a certain kind of neurons (GABAergic medium spiny neurons) in the nucleus accumbens (the reward center in the brain). This occurs through a dopamine D1 selective mechanism. N-acetylcysteine, a drug that can be found in health food stores, decreases cocaine intake in animals and humans, and also normalizes the size of dendritic spines.
Depression
Depression in animals and humans is associated with decreases in Rac1, a protein in the dendritic spines on GABA neurons in the nucleus accumbens. Rac1 regulates actin and other molecules that alter the shape of the spines.
In an animal model of depression called defeat stress, rodents are stressed by repeatedly being placed in a larger animal’s territory. Their subsequent behavior mimics clinical depression. This kind of social defeat stress decreases Rac1 and causes spines to become thin and lose some function. Replacing Rac1 returns the spines to a more mature mushroom shape and reverses the depressive behavior of these socially defeated animals. Researcher Scott Russo has also found Rac1 deficits in the nucleus accumbens of depressed patients who committed suicide. Russo suggests that decreases in Rac1 are responsible for the manifestation of social avoidance and other depressive behaviors in the defeat stress animal model, and that finding ways to increase Rac1 in humans would be an important new target for antidepressant drug development.
Another animal model of depression called chronic intermittent stress (in which the animals are exposed to a series of unexpected stressors like sounds or mild shocks) also induces depression-like behavior and makes the dendritic spines thin and stubby. The drug ketamine, which can bring about antidepressant effects in humans in as short a time as 2 hours, rapidly reverses the depressive behavior in animals and converts the spines back to the larger, more mature mushroom-shape they typically have.
Learning and Extinction of Fear
Researcher Wenbiao Gan has reported that fear conditioning can change the number of dendritic spines. When animals hear a tone paired with an electrical shock, they begin to exhibit a fear response to the tone. In layer 5 of the prefrontal cortex, spines are eliminated when conditioned fear develops, and are reformed (near where the eliminated spines were) during extinction training, when animals hear the tones without receiving the shock and learn not to fear the tone. However, in the primary auditory cortex the changes are opposite: new spines are formed with learning, and spines are eliminated with extinction.
Editor’s Note: It appears that we have arrived at a new milestone in psychiatry. In the field of neurology, changes seen in the brains of patients with strokes or Alzheimer’s dementia have been considered “real” because cells were obviously lost or dead. Psychiatry, in comparison, has been considered a soft science because neuronal changes have been more difficult to see and illnesses were and still are called “mental.” Now that new technologies have made a deeper level of precision, observation, and analysis possible, we know that the brain’s 12 billion neurons and 4 times as many glial cells are exquisitely plastic–capable of biochemical and structural changes that can be reversed using appropriate therapeutic maneuvers.
The changes associated with abnormal behaviors, addictions, and even normal processes of learning and memory now have clearly been shown to correspond with the size, shape, and biochemistry of dendritic spines. These subtle, reproducible changes in the brain and body are amenable to therapeutic intervention, and are now even more demanding of sophisticated medical attention.