Abnormal Levels of Cytokines Found in Brains of Suicide Victims
Cytokines are chemical messengers that send signals between immune cells and between the immune system and the central nervous system. Their levels in blood are considered a measure of inflammation, which has been implicated in depression and stress. A new study by Ghanshyam Pandey and colleagues reported increased levels of cytokines in the brains of people who committed suicide. In the prefrontal cortices of people who died by suicide, there were significantly elevated levels of the inflammatory cytokines IL-1 beta, IL-6 and TNF-alpha compared to the brains of normal controls. There were also lower levels of protein expression of the cytokine receptors IL-1R1, IL-1R2 and IL-1R antagonist (IL1RA) in the suicide brains compared to controls.
The researchers concluded that abnormalities in proinflammatory cytokines and their receptors are associated with the pathophysiology of depression and suicide. This research provides direct confirmation of the indirect measures of inflammation observed in the blood of depressed patients compared to controls.
How the Chemicals in Marijuana Work in the Brain
Raphael Mechoulam, who first synthesized THC, the main ingredient in marijuana, gave the history of marijuana and its receptors in the central nervous system in a plenary talk at the 2014 meeting of the International College of Neuropsychopharmacology. In Syria hundreds of years ago the drug was named ganzigunnu, meaning “the drug that takes away the mind.” It has also been called azalla, meaning “hand of the ghost.” Among the 100 compounds in marijuana, the best-known ingredient is delta-9-tetrahydrocannabinol (delta-9 THC), which produces most of the actions of the drug. There is another active ingredient, cannabidiol (CBD), which has calming and anti-anxiety effects, but is present in very low levels.
The brain has cannabinoid receptors that respond to ingredients in marijuana in addition to other chemicals produced in the brain. They modulate calcium ions and decrease the release of many neurotransmitters.
THC acts at CB-1 receptors, producing the high. The CB-1 receptor is synthesized on demand, post-synaptically, and is transferred to the pre-synaptic terminal where it decreases calcium and transmitter release. Consistent with marijuana’s appetite-stimulating properties (“the munchies”), if the CB-1 receptor is blocked in animals, they lose their appetite and die of hunger.
There are also low levels of CB-2 receptors in the brain, whose activation does not cause a high, and whose levels may increase dramatically in pathological situations. Activation of the CB-2 receptor is anti-inflammatory and, in the same way that the immune system acts against foreign proteins, CB-2 acts as a protector against non-proteins.
CBD does not bind to any cannabinoid receptors, but its actions are blocked by cannabinoid antagonists.
There are two chemicals in the brain (endogenous ligands) that act at cannabinoid receptors—anandamide and 2-arachidonoylglycerol (2-AG). They are soluble only in lipids (not in water), and have never been given to people. In animals, 2-AG has neuroprotective effects, decreases the size of a stroke by 60%, and increases recovery from stroke.
Marijuana and CBD in particular have also had beneficial effects in people. Marijuana decreases the nausea and vomiting associated with chemotherapy in children, has anti-inflammatory effects in rheumatoid arthritis (decreasing inflammatory marker TNF alpha), and has anti-diabetes and anti-convulsant effects.
In 2012, researcher F. Markus Leweke and colleagues showed that CBD was about as effective as the atypical antipsychotic amisulpiride in alleviating the psychotic symptoms of schizophrenia. CBD’s other effects include reducing anxiety and improving psoriasis by increasing DNA methylation (Pucci et al. 2013).
It seems possible that some of these myriad effects of marijuana and endogenous ligands at CB receptors could be exploited for clinical therapeutics, as Mechoulam endorses, but when and how that will take place remains an unanswered question.
Editor’s Note: Despite all these potential positives of CBD, it should be noted that its levels are very low in marijuana, and that heavy smoking of marijuana has substantial adverse effects. These include low motivation, a doubling of the risk of psychosis, a hastening of the onset of bipolar disorder and schizophrenia, and cognitive impairment, as well as some changes in brain structure seen via magnetic resonance imaging (MRI). It may be becoming legal in many states, but is a bad idea for those at high risk for mood, anxiety, or bipolar disorders or for schizophrenia.
Exercise Helps Mice with Spacial Learning
Exercise increases brain-derived neurotrophic factor (BDNF), a protein that protects neurons and is important for learning and memory. In a study of mice who were trained to find objects, sedentary mice could not discriminate between familiar object locations and novel ones 24 hours after receiving weak training, while mice who had voluntarily taken part in exercise over a 3-week period could easily distinguish between these locations after the weak training.
Mice who received sodium butyrate (NaB) after training behaved similarly well to those who had exercised. Sodium butyrate is a histone deacetylase (HDAC) inhibitor, meaning it helps keep acetyl groups on histones, around which DNA is wrapped, making the DNA easier to transcribe. In this case the easy transcription of DNA enables learning under conditions in which it might not usually take place.
Both sodium butyrate and exercise promote learning through their effects on BDNF in the hippocampus. They make the DNA for BDNF easier to transcribe, suggesting that exercise can put the brain in a state of readiness to create new or more lasting memories.
Neurosteroid Allopregnanolone May Improve Bipolar Depression
Sherman Brown of the University of Texas Southwestern reports that the neurosteroid allopregnanolone has positive effects in bipolar depression. Patients in Brown’s study received doses of 100mg capsules twice daily during the first week, then one capsule in the morning and two capsules in the evening during the second week, and two capsules in the morning and three capsules in the evening during the third week.
Neurosteroids can change the excitability of neurons through their interactions with the neurotransmitters that carry signals from neurons across synapses. Among the various types of neurotransmitters, GABA plays an inhibitory role, while glutamate is responsible for excitability. Allopregnanolone, which is naturally produced in the body, has positive effects on GABA receptors and inhibitory effects on glutamate NMDA receptors, so that it increases the balance of inhibition (GABA) over excitation (glutamate).
How Inflammation Increases Glutamate Overexcitation And Neurotoxicity
Research has shown a link between inflammation and mental illness. Inflammation leads to a series of chemical changes that can overexcite neurons and interfere with the protection of neurons.
Inflammation increases the production of indoleamine-pyrrole 2,3-dioxygenase (IDO), an enzyme that breaks down the amino acid tryptophan into kynurenic acid and quinolinic acid. They in turn increase glutamate, the main excitatory neurotransmitter, and decrease brain-derived neurotrophic factor (BDNF), which keeps neurons healthy.
Kynurenic acid stimulates microglia, which clean up the central nervous system as a form of immune defense, to produce inflammatory cytokine proteins.
Quinolinic acid directly stimulates glutamate receptors and encourages glutamate release from astrocytes. Quinolinic acid also blocks glutamate removal that would normally occur through reuptake into the astrocytes, leading to more stimulation of extrasynaptic glutamate receptors and decreases in BDNF.
Quinolinic acid’s effects are opposite to those of the antidepressant ketamine, which blocks glutamate NMDA receptors and increases BDNF. When people are given interferon protein for the treatment of cancers, quinolinic acid increases in cerebrospinal fluid, inducing depression. The severity of depression induced is correlated with the patient’s levels of quinolinic acid.
It appears that ketamine has indirect anti-inflammatory effects through its ability to block glutamate receptors and increase BDNF.
Lithium Reverses Effects of Oxidative Stress on Mitochondrial Function
Oxidative stress has been implicated in a wide range of illnesses, but what is it exactly? Our bodies use the oxygen we breathe to burn the fuel we get from food, and while this is a natural process, it produces byproducts known as free radicals, which are unstable molecules that can strip electrons from other molecules in a process called oxidation. Antioxidants (such as vitamin C) act as a source of electrons, helping keep other cells stable and healthy. Oxidative stress refers to the stress on our bodies from the normal effects of free radicals combined with environmental stressors like tobacco smoke or radiation.
In work presented at the 2013 meeting of the Society of Biological Psychiatry, Anna Andreason showed that over-activity of neurons increases oxidative stress through the production of reactive oxygen species (ROS). These are a type of free radicals that can damage cells in two ways: nitrosylation of proteins (adding nitric oxide to a thiol molecule), and oxidation, which results in more lasting effects on synaptic structures. The chemical compound rotenone damages mitochondria by producing ROS, and Andreason found that lithium was able to reverse this production and reverse the adverse effects of oxidative stress.
Lithium Has an Amazing Array of Positive Effects
Editor’s Note: The ability of lithium to protect mitochondria (the energy storehouse of a cell) adds to an increasingly long list of lithium’s neurotropic and neuroprotective benefits. Lithium increases cell survival factors BDNF and Bcl-2, increases markers of neuronal integrity such as N-Acetylaspartic acid (NAA), increases the volume of the hippocampus and cortex, and now helps protect mitochondria from oxidative stress. Lithium also increases the length of telomeres, which cap the ends of chromosome and protect them from damage during the DNA replication that occurs each time a cell divides. Short telomeres are associated with many kinds of medical and psychiatric diseases, as well as shorter life spans. No wonder that in addition to preventing mania and depression it has other clinical benefits, such as preventing memory deterioration, medical mortality, and suicide.
Neuronendocrine Function in Sexually Abused Adolescent Girls
Brooks R. Keeshin from a research group led by Frank Putnam presented a poster at the 2012 meeting of the American Academy of Child and Adolescent Psychiatry (AACAP) on neuroendocrine function in recently sexually abused adolescent girls with and without post-traumatic stress disorder (PTSD). Average age of the girls was 15 and they had experienced the sexual abuse six months to one year before the study. The researchers found that morning cortisol awakening response was flattened in the girls, and this was associated with PTSD severity and the severity of intrusive symptoms. Increased adversity prior to the sexual abuse experience was also associated with flattening of the cortisol awakening response.
The researchers suggest that alterations in the hypothalamic pituitary adrenal axis (HPA) appear around the time of the abuse and are associated with the severity of PTSD symptomatology in sexually abused adolescent girls.
Rare Encephalitis Affects NMDA Receptor
At the 57th Annual Meeting of the American Academy of Child and Adolescent Psychiatry (AACAP) in October 2010, Nadine Schwartz reported that in a rare type of encephalitis, antibodies specifically target and bind to N-methyl-D-aspartate (NMDA) receptors, the major receptors for excitatory neurotransmission in the brain. Individuals usually develop psychiatric symptoms before neurological ones, and were previously often thought to be malingering or inventing their illness. Eventually they may develop profound cognitive and motor deterioration and many may require treatment in an intensive care unit in order to provide adequate respiration. Studies show that children with this syndrome appear to respond to anti-immune therapeutic approaches including steroids, plasmaphoresis, and antimetabolites.
Editor’s Note: The recognition that auto-antibodies can attack the major receptors for excitatory neurotransmission in brain brings to light another potential mechanism that could explain neurochemical dysregulation in the neuropsychiatric disorders.
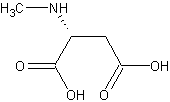
N-acetylcysteine Affects Electrical Activity in Brain
At the 65th Annual Scientific Convention of the Society of Biological Psychiatry, researcher Christian Carmeli reported that N-acetylcysteine (NAC, 2 gm/day for six months) increased electroencephalogram (EEG) synchrony over the frontal cortical and left temporal regions in patients with schizophrenia. The EEG measures the frequency and amplitude of electrical activity on both sides of the brain.
Editor’s note: These data provide a neurophysiological mechanism that could explain the positive effects of NAC previously observed by researcher Mike Berk and associates in both schizophrenia and bipolar disorders and published in Biological Psychiatry in 2008. NAC is both a glutathione precursor providing antioxidant effects and a modulator of hyper-responsive glutamate reactivity in the n. accumbens or ventral striatum, the reward area of the brain. In placebo-controlled studies NAC appears effective in treating cocaine, heroin, and gambling addiction, as well as trichotillomania (compulsive hair-pulling). These effects are thought to be related to NAC’s dampening of glutamate responses in the n. accumbens, which, along with the dorsal striatum, appears to mediate habit memory. Read more
Episodic vs. Continuous Social Stress Result in Different Rates of Cocaine Use
In a study of rodents exposed to stress (by being forced to enter another rodent’s territory) and given the opportunity to self-administer cocaine, those exposed to a few brief episodes of stress increased their cocaine use and engaged in binge-like episodes, while those exposed to stress chronically showed suppressed cocaine use.
At the American College of Neuropsychopharmacology meeting in December 2009, Klaus Miczek and colleagues from Tufts University in Boston presented a fascinating study indicating that the temporal aspects of the experience of social stress may have dramatic impact not only on defeat stress behaviors and the associated biochemistry, but also on the likelihood that an animal adopts cocaine self-administration. These investigators compared episodic versus chronic defeat stress in rodents.
Episodic social defeat stress consisted of four brief confrontations between an intruding animal and an aggressive resident rat over the course of a period of ten days. In contrast, chronic subordination stress involved the continuous exposure of the intruder rat to an aggressive resident over five weeks, during which time the intruder lived in a protective cage within the resident’s home cage.
The episodically defeated intruder rats showed increases in intravenous cocaine self-administration and prolonged binge-like episodes, along with increases in brain-derived neurotropic factor (BDNF), which is necessary for long-term learning and memory, in the midbrain ventral-tegmental area (VTA) and increased dopamine release in the nucleus accumbens, the reward area of the brain. In contrast, the continuously subordinate rats showed the opposite pattern of suppressed cocaine intake, suppression of dopamine release in the n. accumbens, and reduced BDNF in the VTA.





-N-Acetylcysteine_Structural_Formulae.png)
