DNA Repair Plays Role in Brain Development, Cancer, and Aging
DNA has several ways of repairing itself. Serious damage, including breaks to both strands of the double helix and problems with replication, prompt a process known as DNA damage repair, or DDR. Researcher Stephen J. Elledge of Harvard Medical School won the 2015 Albert Lasker Basic Medical Research Award for his findings about DDR. He summarized these findings in a September article in the journal JAMA.
DDR occurs because of DNA’s remarkable self-awareness. Through the DDR process, DNA can detect when it has been damaged and prompt the right kind of repair. When damage occurs, DDR allows for the activation of enzymes that can remodel DNA to maintain the integrity of the genome.
When DDR pathways are activated, they can alter more than 1000 different proteins. DDR can affect immune function, blood and bone marrow, viral response, cancer, aging, and brain development.
Mutations in components of the DDR pathway can lead to problems with brain development, including Seckel syndrome (characterized by dwarfism, brain and facial abnormalities, and mental retardation) and ataxia telangiectasia (loss of control of bodily movements along with weakened immune system).
DDR is particularly relevant to cancer, since properly functioning DDR promotes a stable genome. Classic cancer treatments such as radiation and chemotherapy also rely on DDR to prompt cell death.
DDR also plays a role in aging. When we get older or have certain illnesses, telomeres, bits of material at the end of DNA strands that protect the DNA during replication, get shorter. This prompts DDR to engage in tumor prevention measures, either killing off the cells or changing them into what’s called senescent cells. Senescent cells prevent tumors, but their accumulation is associated with chronic inflammation, aging, and age-related diseases.
Editor’s Note: You can protect your telomeres and possibly hold off the age-related effects of DDR. Healthy diet, exercise, meditation, goal setting, and making positive contributions to society all help maintain telomere length. Lithium treatment also directly increases telomere length.
In Rats, Dad’s Cocaine Use Affects Son’s Spatial Memory
Evidence is mounting that certain behaviors by parents can leave marks on their sperm or eggs that are passed on to their offspring in a process called epigenetics. In a recent study by researcher Mathieu Wimmer and colleagues, male rats that were exposed to cocaine for 60 days (the time it takes for sperm to develop fully) had male offspring who showed diminished short- and long-term spatial memory compared to the offspring of male rats that were not exposed to cocaine. Female offspring were not affected in this way.
The spatial tasks the offspring rats completed depended heavily on the hippocampus. Wimmer and colleagues believe that cocaine use in the fathers decreased the amount of a brain chemical called d-serine in the offspring. D-serine plays a role in memory formation and the brain’s ability to form synaptic connections. Injecting the offspring of rats who were exposed to cocaine with d-serine before the spatial memory tasks normalized the rats’ performance.
Over-Pruning of Synapses May Explain Schizophrenia
A gene that plays a role in the pruning of synapses has been linked to schizophrenia. The gene encodes an immune protein called complement component 4 (C4), which may mediate the pruning of synapses, the connections between neurons. Researchers led by Aswin Sekar found that in mice, C4 was responsible for the elimination of synapses. The team linked gene variants that lead to more production of C4A proteins to excessive pruning of synapses during adolescence, the period during which schizophrenia symptoms typically appear. This may explain why the brains of people with schizophrenia have fewer neural connections. The researchers hope that future therapies may target the genetic roots of the illness rather than simply treating its symptoms.
Memory Activates Epigenetic Changes in Mice Brain Cells
In a 2015 article in Nature Neuroscience, Stefan Bonn and André Fischer reported that when mice were prompted to use their long-term memory to recognize a specific environment, epigenetic changes occurred in their neurons and glia. Epigenetic changes refer to chemical alterations in DNA or histones (which give DNA structure) that increase or decrease the expression of certain genes. Sometimes environmental factors lead to a methyl or acetyl group joining a strand of DNA or histones, changing how easily the genes are turned on or off.
When the mice used their long-term memory, the main change that occurred was DNA methylation in their neurons. There were also changes to histones that were linked to memory acquisition but resulted in few changes in gene expression. The DNA methylation changes, on the other hand, changed neural pathways, leading to “rewiring” of the brain.
Link Between Childhood Trauma and Difficult Course of Bipolar Disorder Clarified
A collaboration between Norwegian and French researchers led by Bruno Etain has clarified the pathway by which childhood trauma is linked to worse outcomes among people with bipolar disorder. The researchers, who presented their work in a poster at the 2015 meeting of the Society of Biological Psychiatry, replicated earlier findings by this editor (Robert Post) that patients who experienced trauma as a child had a more adverse course of bipolar disorder. Etain and colleagues found a link between childhood trauma and an earlier age of onset of bipolar disorder, rapid cycling, suicide attempts, and cannabis misuse.
The researchers identified more than 550 patients with bipolar disorder, who answered questionnaires about their history of bipolar disorder and childhood trauma. Their DNA was also analyzed, and the researchers found that the effect of childhood trauma on age of onset was mediated by the presence of common genetic variants in proteins related to stress (the serotonin transporter) and immune function (Toll-like receptors). They also found that the traits of mood lability (or moodiness) and impulsivity mediated the effects of trauma on clinical outcomes.
The lasting epigenetic effects of child maltreatment and adversity noted in the above abstract are consistent with a large literature showing more epigenetic effects in these individuals than in controls. While genetics are important, the impact of the environment is also substantial.
Early Experiences Have Lasting Effects on DNA
It is well established that certain early experiences can affect a person’s risk of developing a mental illness. Adversity in childhood, including abuse or the loss of a parent, is a risk factor not only for diagnosis of a mood disorder, but also for a more difficult course of illness. This may occur through epigenetic means. Epigenetics refers to a process by which environmental factors can change the way that DNA is transcribed, for example through the addition of methyl groups to strands of DNA. This tends to inhibit DNA from being transcribed and producing protein growth factors and other neurochemicals that are important for development.
A study by Kieran J. O’Donnell and colleagues presented at the 2015 meeting of the Society of Biological Psychiatry investigated whether epigenetics play a role in the success of a parenting intervention called the Nurse Family Partnership. Participants were 27-year-olds born to women who had received the intervention or a control intervention. Genome-wide DNA methylation was measured in the 188 participants’ blood.
Analysis of the blood revealed that the Nurse Family Partnership intervention was associated with DNA methylation at 1015 sites across 593 genes. Some of these sites were enriched for certain neurodevelopmental processes. Maltreatment in childhood was also associated with methylation at 1552 sites across 878 genes.
Editor’s Note: The take-home message of this landmark study is that maltreatment in childhood exerts lasting effects on the genome via epigenetic mechanisms, but early positive intervention also exerts lasting epigenetic effects, which likely have a normalizing impact.
A Note on Genetic Inheritance
Genetic inheritance is not everything, according to J. Craig Venter, pioneering genetic scientist responsible for sequencing the human genome in 2001:
“Human biology is actually far more complicated than we imagine. Everybody talks about the genes that they received from their mother and father, for this trait or the other. But in reality, those genes have very little impact on life outcomes. Our biology is far too complicated for that and deals with hundreds of thousands of independent factors. Genes are absolutely not our fate. They can give us useful information about the increased risk of a disease, but in most cases they will not determine the actual cause of the disease, or the actual incidence of somebody getting it. Most biology will come from the complex interaction of all the proteins and cells working with the environmental factors, not driven directly by the genetic code.”
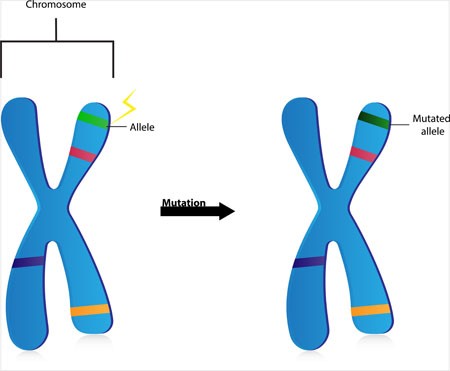
“De Novo” Mutations in Dozens of Genes Cause Autism
Two studies that incorporated data from more than 50 labs worldwide have linked mutations in more than 100 different genes to autism. Scientists have a high level of statistical confidence that mutations in about 60 of those genes are responsible for autism. So-called de novo mutations (Latin for “afresh”) do not appear in the genes of parents without autism, but arise newly in the affected child. The mutations can alter whether the genes get “turned on” or transcribed (or not), leading to disturbances in the brain’s communication networks.
The studies led by Stephan Sanders and Matthew W. State appeared in the journal Nature in late 2014. The identified genes fall into three categories. Some affect the formation and function of synapses, where messages between neurons are relayed. Others affect transcription, the process by which genes instruct cells to produce proteins. Genes in the third category affect chromatin, a sort of packaging for DNA in cells.
Before the new studies, only 11 genes had been linked to autism, and the researchers involved expect to find that hundreds more are related to the illness.
Editor’s Note: This new research explains how autism could be increasing in the general population even as most adults with autism do not have children. It should also put to rest the idea, now totally discredited, that ingredients in childhood immunizations cause autism. It is clearer than ever that kids who will be diagnosed with autism are born with these mutations.
With these genetic findings, the search for new medications to treat this devastating illness should accelerate even faster.
Bottom line: Childhood immunizations don’t cause autism, newly arising mutations in the DNA of parents’ eggs or sperm do. However, parental behavior could put their children and others at risk for the measles and other serious diseases if they do not allow immunizations. The original data linking autism to immunization were fraudulent, and these new data on the genetic origins of autism provides the best hope for future treatments or prevention.
Genetic Variation Predicts Which Type of Antidepressant Will Be Effective
 In a six-month study of Caucasian patients, normal variations in the gene that is responsible for brain-derived neurotrophic factor (BDNF) predicted whether patients would respond better to a selective serotonin reuptake inhibitor (SSRI) antidepressant versus a serotonin and norepinephrine reuptake inhibitor (SNRI) or a tricycle antidepressant. There are several common variants of the BDNF gene, depending on which types of amino acids appear in its coding—valine or methionine. Patients with the most common version, two valines (or Val66Val), responded better to SSRIs. About two-thirds of the population has this version of the gene, which functions most efficiently. The remaining third have at least one methionine in the BDNF gene. Patients with a Met variation responded better to SNRIs and tricyclic antidepressants.
In a six-month study of Caucasian patients, normal variations in the gene that is responsible for brain-derived neurotrophic factor (BDNF) predicted whether patients would respond better to a selective serotonin reuptake inhibitor (SSRI) antidepressant versus a serotonin and norepinephrine reuptake inhibitor (SNRI) or a tricycle antidepressant. There are several common variants of the BDNF gene, depending on which types of amino acids appear in its coding—valine or methionine. Patients with the most common version, two valines (or Val66Val), responded better to SSRIs. About two-thirds of the population has this version of the gene, which functions most efficiently. The remaining third have at least one methionine in the BDNF gene. Patients with a Met variation responded better to SNRIs and tricyclic antidepressants.
The study by R. Colle and colleagues was published in the Journal of Affective Disorders in 2015. Of the patients who were prescribed SSRIs, 68.1% of patients with the Val/Val version responded to the medication after three months, compared to 44% of the patients with a Met version. Of patients prescribed SNRIs or tricyclics, 60.9% of the Met patients reached remission by six months, compared to only 33.3% of the Val/Val patients.
Editor’s Note: In an earlier BNN we reported that according to research published by Gonzalo Laje and colleagues in the journal Biological Psychiatry in 2012, depressed patients with the better functioning Val66Val allele of BDNF respond best to ketamine, while those with the intermediate functioning Val66Met allele respond less well.
Gene CACNA1C is Associated with Early-Onset Bipolar Disorder
Several genes have previously been implicated in bipolar illness. In a recent study, researchers at the Mayo Clinic, led by Paul Croarkin, compared variations in three genes (CACNA1C, ANK3, and ODZN) across 69 children aged 6–15 with mania, a 776-person control group from the Mayo Biobank database, and 732 adults with bipolar disorder (some with onset in childhood and adolescence and some with onset in adulthood, also from the Biobank). All participants were Caucasian, to minimize confounding by population stratification. The researchers found that the minor allele of rs10848632 in CACNA1C was associated with childhood onset of bipolar disorder. The haplotype (or sequence of nucleotides) T-G-G-T was the one associated with risk. Genetic risk scores were also associated with early onset of illness.
Editor’s Note: In research by Michael McCarthy and colleagues, CACNA1C has been linked to abnormal circadian rhythms in bipolar disorder and to responsiveness to lithium treatment. Together, these data suggest the importance of studying the calcium channel blocker nimodipine (which blocks calcium influx through CACNA1C) in childhood-onset bipolar disorder. A 1999 case report by Pablo A. Davanzo and colleagues described a teenager with ultra rapid cycling bipolar disorder (multiple mood switches/day) that did not respond to a host of conventional medications, who improved dramatically on nimodipine, reaching remission. This author (Robert M. Post) has also seen confirmed responsivity in adults with rapid cycling bipolar disorder (reported in the 2008 book Treatment of Bipolar Illness: A Casebook for Clinicians and Patients, by Post and Gabriele S. Leverich).