Inflammation Plays a Role in Fear Memories
People with post-traumatic stress disorder (PTSD) often experience fearful memories of the trauma they witnessed. Researchers are working to determine the neurobiological basis for these persistent fear memories in order to better treat PTSD. Current treatments mainly target the central nervous system. Because many people with PTSD have elevated levels of pro-inflammatory immune molecules in their blood, there has been a recent push to determine whether targeting that inflammation may be another way of treating PTSD.
A recent study by researchers Matthew Young and Leonard Howell used an animal model to learn more about the link between trauma, inflammation, and fear memories. The researchers exposed mice to a trauma that produced both a persistent fear response and an increase in inflammatory molecules in the blood. Some of the mice were also given antibodies to neutralize the inflammatory immune response. When the mice were exposed to a cue meant to remind them of the trauma, levels of the inflammatory molecule IL-6 spiked again. When the mice were given antibodies to neutralize IL-6 just before being exposed to the cue, they produced less of a fear reaction.
The researchers, who presented their work at a scientific meeting in December, concluded that traumatic experiences produce not only persistent fearful memories, but also an immune reaction. They believe that the spike in IL-6 following trauma plays a role in the persistence of those memories, and that elevated IL-6 in the blood may explain symptoms of PTSD and other disorders that involve fear learning (such as phobias).
Some Evidence of Brain Inflammation in Depression
 Many studies have found links between levels of inflammatory molecules in the blood and depression or depressive symptoms. There has been less research about inflammation in the brain and its possible role in depressive illness. Improvements in positron emission topography (PET) scan technology now allow for better brain imaging that can reveal when microglia are activated. (Microglia serve as the main immune responders in the central nervous system.)
Many studies have found links between levels of inflammatory molecules in the blood and depression or depressive symptoms. There has been less research about inflammation in the brain and its possible role in depressive illness. Improvements in positron emission topography (PET) scan technology now allow for better brain imaging that can reveal when microglia are activated. (Microglia serve as the main immune responders in the central nervous system.)
A study by researcher Jeffrey Meyer found evidence of microglial activation in several brain regions (including the prefrontal cortex, the anterior cingulate cortex, and the insula) in people in an episode of depression who were not receiving any treatments. Participants with more microglial activation in the anterior cingulate cortex and insula had more severe depression and lower body mass indexes.
Meyer, who presented this research at a scientific meeting in December, called it strong evidence for brain inflammation in depressive episodes, and suggested that treatments that target microglial activation would be promising for depression.
However, at the same meeting, researcher Erica Richards reported that she had not been able to replicate Meyer’s results. Her research, which included depressed participants both on and off medication and non-depressed participants, found that depressed participants did show more inflammation in the two brain regions she targeted, the anterior cingulate and the subgenual cortices, but this difference did not reach statistical significance, particularly when patients taking antidepressants were included in the calculations. Richards hopes that with a greater sample size, the data may show a significant difference in brain inflammation between depressed and non-depressed participants.
Inflammation Linked to Bipolar Illness in Young People
 The Course and Outcome of Bipolar Youth study, or COBY, has been collecting information on young people with bipolar disorder and tracking their symptoms into adulthood since 2000. A 2015 study by Benjamin I. Golstein in the Journal of Clinical Psychiatry analyzed COBY data, identifying links between higher than average levels of inflammatory markers measured in the blood and participants’ histories of illness and familial risk factors.
The Course and Outcome of Bipolar Youth study, or COBY, has been collecting information on young people with bipolar disorder and tracking their symptoms into adulthood since 2000. A 2015 study by Benjamin I. Golstein in the Journal of Clinical Psychiatry analyzed COBY data, identifying links between higher than average levels of inflammatory markers measured in the blood and participants’ histories of illness and familial risk factors.
High levels of the inflammatory marker hsCRP were associated with longer duration of illness, substance use disorder, and family history of suicide attempts or completed suicides. High levels of TNF-alpha were linked to suicide attempts, self-injury behaviors, and family history of substance use disorders. IL-6 was also linked to family history of substance use disorders.
There were also links between inflammatory markers and participants’ symptoms over the 6 months leading up to the blood tests. Levels of the inflammatory marker TNF-alpha were linked to the percentage of weeks patients had psychotic symptoms. Levels of IL-6 were associated with percentage of weeks with subthreshold mood symptoms and also with any suicide attempt. Levels of HsCRP were linked to maximum severity of depressive symptoms.
It is possible that targeting the elevated levels of inflammatory markers with anti-inflammatory treatments could improve patients’ response to treatments, but this topic requires further study.
L-methylfolate Improves Depression; More Effective in Overweight Patients with Inflammation
A 2012 study by Geoge I. Papkostas and colleagues found that 15mg/day of the nutritional supplement l-methylfolate calcium (a form of the B vitamin folate that the body can more readily use) improved depression in people who had not responded adequately to treatment with a selective serotonin reuptake inhibitor (SSRI) antidepressant. In a follow-up study by Richard C. Shelton and colleagues published in the Journal of Clinical Psychiatry in 2015, the same researchers further analyzed these data and found that l-methylfolate worked better in patients who were overweight (with body mass indexes (BMIs) of 30 or above) and those who had higher than average levels of inflammation at the beginning of the study. Inflammatory markers linked to greater improvement with l-methylfolate included TNF-alpha, IL-8, high sensitivity CRP, and leptin. In overweight participants, higher than average levels of IL-6 were also linked to more improvement on l-methylfolate.
Brain Inflammation in People at High Risk for Schizophrenia
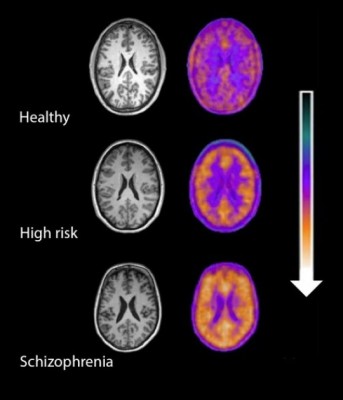
Microglial activity in the brains of people who are healthy, people at high risk for schizophrenia, and people who have been diagnosed with schizophrenia.
A 2016 study by Peter S. Bloomfield and colleagues in the American Journal of Psychiatry used PET scans to compare the activity of microglia, immune cells in the central nervous system, in healthy controls, people with schizophrenia, and those at high risk for the illness. It found that both people with schizophrenia and those at high risk had greater brain inflammation than the healthy controls.
The study was the first to show that microglial activity was elevated in people at high risk (who showed some preliminary symptoms of schizophrenia). The finding had a large effect size.
Microglial activity was also correlated with symptom severity in the high-risk participants. Increased microglial activity was not linked to depression, suggesting that it is specific to the development of psychosis.
These findings resemble those of other recent studies showing increased inflammation in people at high risk for psychosis.
The study suggests that increased microglial activity occurs before a first episode of psychosis. That means it could help identify people who may develop schizophrenia. The findings also suggest that anti-inflammatory treatment could theoretically be used to prevent psychosis.
Reduced Cognitive Function and Other Abnormalities in Pediatric Bipolar Disorder
 At the 2015 meeting of the International Society for Bipolar Disorders, Ben Goldstein described a study of cognitive dysfunction in pediatric bipolar disorder. Children with bipolar disorder were three years behind in executive functioning (which covers abilities such as planning and problem-solving) and verbal memory.
At the 2015 meeting of the International Society for Bipolar Disorders, Ben Goldstein described a study of cognitive dysfunction in pediatric bipolar disorder. Children with bipolar disorder were three years behind in executive functioning (which covers abilities such as planning and problem-solving) and verbal memory.
There were other abnormalities. Youth with bipolar disorder had smaller amygdalas, and those with larger amygdalas recovered better. Perception of facial emotion was another area of weakness for children (and adults) with bipolar disorder. Studies show increased activity of the amygdala during facial emotion recognition tasks.
Goldstein reported that nine studies show that youth with bipolar disorder have reduced white matter integrity. This has also been observed in their relatives without bipolar disorder, suggesting that it is a sign of vulnerability to bipolar illness. This could identify children who could benefit from preemptive treatment because they are at high risk for developing bipolar disorder due to a family history of the illness.
There are some indications of increased inflammation in pediatric bipolar disorder. CRP, a protein that is a marker of inflammation, is elevated to a level equivalent to those in kids with juvenile rheumatoid arthritis before treatment (about 3 mg/L). CRP levels may be able to predict onset of depression or mania in those with minor symptoms, and is also associated with depression duration and severity. Goldstein reported that TNF-alpha, another inflammatory marker, may be elevated in children with psychosis.
Goldstein noted a study by Ghanshyam Pandey that showed that improvement in pediatric bipolar disorder was related to increases in BDNF, a protein that protects neurons. Cognitive flexibility interacted with CRP and BDNF—those with low BDNF had more cognitive impairment as their CRP increased than did those with high BDNF.
Maternal Infection During Pregnancy May Increase Risk of Schizophrenia in Offspring
 There is mounting evidence from animal studies and epidemiological research that an infection during pregnancy may increase the risk of schizophrenia in the offspring. A recent study by Alan Brown and colleagues presented at the 2015 meeting of the Society of Biological Psychiatry used a large dataset from the Finnish Prenatal Study of Schizophrenia to compare medical data from the mothers of 777 people with schizophrenia (630 with schizophrenia and 147 with schizoaffective disorder) to data from the mothers of 777 healthy people.
There is mounting evidence from animal studies and epidemiological research that an infection during pregnancy may increase the risk of schizophrenia in the offspring. A recent study by Alan Brown and colleagues presented at the 2015 meeting of the Society of Biological Psychiatry used a large dataset from the Finnish Prenatal Study of Schizophrenia to compare medical data from the mothers of 777 people with schizophrenia (630 with schizophrenia and 147 with schizoaffective disorder) to data from the mothers of 777 healthy people.
The study’s biobank contained blood samples taken from the mothers in early to mid-pregnancy, which the researchers used to determine the mothers’ levels of C-reactive protein (CRP), an indicator of inflammation. Higher levels of CRP were associated with increased risk of schizophrenia in the offspring. When the researchers analyzed the findings by sex of the offspring, the link between prenatal infection and schizophrenia risk was significant in males, but not females. The effect was also stronger among offspring born after their due date than those born at or before their due date.
Maternal Infection in Mice Leads to Three Generations of Behavioral Changes
Epigenetics is the process by which environmental factors affect the way a person’s genes are transcribed. These changes, which may include the addition or subtraction of methyl groups from DNA, change the DNA’s structure (how tightly it is wound around the histones that give it shape) but not its sequence. These structural changes, which affect how easily the DNA is transcribed, can then be passed on to future generations. A new study by Ulrike Stadlbauer and colleagues presented at the Society of Biological Psychiatry explored a particular pathway by which an infection in a pregnant mouse can lead to behavioral changes in three following generations of mice.
Pregnant mice were given injections that produced an infection. A first generation of offspring were interbred to create a second generation of offspring, and these were interbred to create a third generation of offspring. The first generation of offspring had epigenetic changes in methylation and hydroxymethylation to promoter regions of two enzymes that regulate synthesis of the neurotransmitter GABA, and these epigenetic changes were associated with reduced mRNA expression of these two genes.
All three generations of offspring had deficits in social interaction, short-term memory, and cued fear conditioning. Interestingly, the second and third offspring generations also exhibited depression-like behavior that had not been present in the original mothers or the first generation of offspring.
Editor’s Note: This is another fascinating demonstration of how environmental occurrences, which can include stressors, exposure to drugs, and now immune challenges, can have effects across generations, likely through epigenetic changes that persist in ova or sperm. Amazingly, it turns out that the environment can change traits in future generations, not by inducing changes to gene sequences, but through epigenetic changes to the structure of DNA or histones that persist across generations.
Preliminary Evidence That Anti-Inflammatory Celecoxib Helps in Bipolar Depression
A study currently in progress indicates that the anti-inflammatory COX-2 inhibitor celecoxib (better known as the arthritis treatment Celebrex) may aid in the treatment of bipolar depression. In a panel session on inflammation at the 2015 meeting of the Society of Biological Psychiatry, researcher Angelos Halaris reported results from the first 26 participants.
Participants were taking mood stabilizers for bipolar disorder and became depressed. They received either 20mg/day of the selective serotonin reuptake inhibitor antidepressant escitalopram (Lexapro) plus either 200mg twice a day of celecoxib or placebo for a total of eight weeks. Those participants who received celecoxib showed greater and more rapid reductions in depression symptoms than those who received placebo.
The study will continue, and Halaris and colleagues will also observe whether measures of inflammation in patients’ blood are correlated with the patients’ responsiveness to the combined treatment with escitalopram and celecoxib.
Blood and Now Brain Inflammation Linked to Depression
 There is growing evidence of a link between inflammation of depression. At the 2015 meeting of the Society of Biological Psychiatry, researcher Jeff Meyer summarized past studies on inflammatory markers. These are measurements, for example of certain proteins in the blood, that indicate the presence of inflammation in the body.
There is growing evidence of a link between inflammation of depression. At the 2015 meeting of the Society of Biological Psychiatry, researcher Jeff Meyer summarized past studies on inflammatory markers. These are measurements, for example of certain proteins in the blood, that indicate the presence of inflammation in the body.
Common inflammatory markers that have been linked to depression include IL-6, TNF-alpha, and c-reactive protein. At the meeting, Meyer reviewed the findings on each of these. Twelve studies showed that IL-6 levels are elevated in the blood of patients with depression. Four studies had non-significant results of link between IL-6 and depression, and Meyer found no studies indicating that IL-6 levels were lower in those with depression. Similarly, for TNF-alpha, Meyer found 11 studies linking elevated TNF-alpha with depression, four with non-significant results, and none showing a negative relationship between TNF-alpha and depression. For c-reactive protein, six studies showed that c-reactive protein was elevated in people with depression, six had non-significant results, and none indicated that c-reactive protein was lower in depressed patients.
Most studies that have linked inflammation to depression have done so by measuring inflammatory markers in the blood. It is more difficult to measure inflammation in the brain of living people, but Meyer has taken advantage of new developments in positron emission tomography (PET) scans to measure translocator protein binding, which illustrates when microglia are activated. Microglial activation is a sign of inflammation. Translocator protein binding was elevated by about 30% in the prefrontal cortex, anterior cingulate cortex, and insula in study participants who showed symptoms of a major depressive episode compared to healthy control participants. The implication is that the depressed people with elevated translocator protein binding have more brain inflammation, probably via microglial activation.
The antibiotic minocycline reduces microglial activation. It would be interesting to see if minocycline might have antidepressant effects in people with depression symptoms and elevated translocator protein binding.





