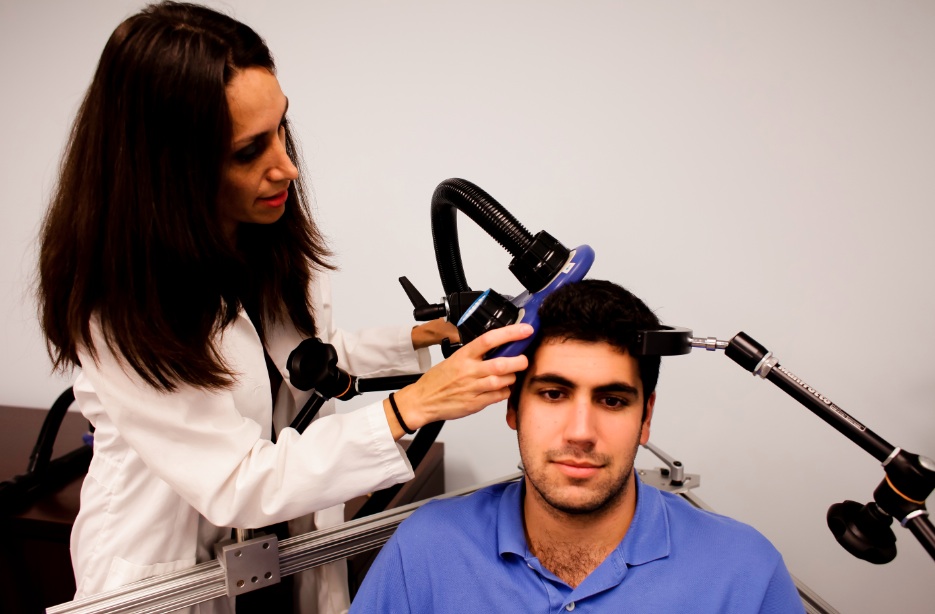
RTMS in Depression: Positive Effects in Long-Term Follow-Up
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive treatment that uses a rapidly changing magnetic field to target neurons, creating a weak electric current. It is used to treat depression, strokes, and other neurological and psychiatric conditions. At the 2013 meeting of the American Psychiatic Association (APA), researcher Linda L. Carpenter reported on new findings about the long-term effects of rTMS.
In the new research, which was led by Mark Andrew Demitrack, 307 patients with unipolar depression who had not responded well to previous antidepressant treatment were given rTMS. They were treated in 43 different clinical practices and administered rTMS according to their evaluating physician, following FDA guidelines. Of the 307 patients who began the study, 264 benefited from initial treatments with rTMS, were tapered off rTMS, and agreed to participate in a one-year follow-up period, by the end of which 68% had improved and 40.4% had achieved complete remission as measured by the Clinical Global Impressions scale for severity of illness.
However, some study participants (30.2%) had worsened by the first follow-up assessment at the 3-month mark, and rTMS had to be re-introduced.
Statin Benefits for Mood, Brain, and Heart Seem to Outweigh Diabetes Risk
 Statins are a class of drugs that are the most commonly prescribed treatment for high cholesterol. They can reduce risk of heart attack and stroke in people with a history of cardiovascular disease. New research is beginning to clarify statins’ other effects, which on the negative side can include increased risk of diabetes and liver and muscle inflammation, and on the positive side can include reduced risk of cataracts and prevention of depression and dementia.
Statins are a class of drugs that are the most commonly prescribed treatment for high cholesterol. They can reduce risk of heart attack and stroke in people with a history of cardiovascular disease. New research is beginning to clarify statins’ other effects, which on the negative side can include increased risk of diabetes and liver and muscle inflammation, and on the positive side can include reduced risk of cataracts and prevention of depression and dementia.
In late 2012, the American Heart Association Scientific Sessions included a discussion of five new studies suggesting that the cardiovascular benefit of taking statins is worth the slightly increased risk of diabetes. Researchers at the conference explained that cardiovascular events are much more serious than the small increase in risk of diabetes. While all five studies showed an increase in diabetes risk, the absolute increase was low and depended on the patients’ level of risk prior to treatment and how high their doses of statins were. There are strategies that can reduce diabetes risk in statins users, including using bile-acid sequestrants, reducing niacin, and monitoring glucose. Consensus at the conference was that statins’ cardiovascular benefits are so important that the drugs shouldn’t be avoided because of concerns about diabetes.
In addition, statins’ beneficial effects on mood have been reported for several years. In 2010, an epidemiological study by Pasco et al. in Psychotherapy and Psychosomatics showed that subjects without depression were less likely to develop a new onset of depression if they were treated with statins compared to those who were not. Stafford et al. reported in the Journal of Clinical Psychiatry in 2010 that patients taking statins had a 79% decreased likelihood of depression at 9 months of follow-up. Moreover, a 2012 meta-analysis by O’Neil et al. in BMC Medicine reported that overall, statins had positive effects on mood.
A recent huge Taiwanese study of statins suggests that the drugs can also prevent dementia. At the European Society of Cardiology congress in 2013, Tin-Tse Lin reported that among 58,000 people studied, those taking the highest dosage of statins had a threefold decrease in risk of developing pre-senile and senile dementia. He explained that it was the potency of statins such as atorvastatin and rosuvastatin that provided the cognitive benefit. However it is high doses that lead to less benign side effects such as liver and muscle inflammation.
A separate US study presented at the congress showed that statin use also lowered risk of developing cataracts by 19%.
Preventing Cognitive Decline in Bipolar Disorder
Here are some suggestions from BNN Editor-in-Chief Robert M. Post, MD for preventing cognitive decline in patients with bipolar disorder.
1. Prevent Episodes with Long-term Prophylaxis
2. Remove Sedating or Impairing Drugs
3. Add Folate to Decrease Homocysteine
4. Treat Depression to Remission
5. Consider Adding:
a. Bupropion (Wellbutrin) for Mood and ADHD
b. Modafinil (Provigil) for Attention and ADHD
c. A Stimulant for ADHD
6. Add Lithium at 150mg/day for Neuroprotection in Mild Cognitive Impairment
7. Add Levitiracetam at 125mg/day to Decrease Hippocampal Hyperactivity
8. Treat Dementia Symptoms Early with:
a. Memantine (Namenda) AND/OR
b. Acetylcholine Esterase Inhibitors Such As Donepezil (Aricept)
9. Consider Adding an Anti-inflammatory Agent
Lithium Increases Hippocampal Volume
 There is some evidence that lithium can affect brain structure, particularly the size of various parts of the brain. A study by Hajek et al. presented at the 2013 meeting of the International Society of Bipolar Disorders examined patients with bipolar disorder who had either received lithium for at least two years (37 patients) or had received under three months of treatment with lithium (19 patients), and compared the size of the hippocampus in these two groups and one control group (50 people). The patients with bipolar disorder all had the disorder for at least 10 years (25 years on average) and had had a minimum of five episodes.
There is some evidence that lithium can affect brain structure, particularly the size of various parts of the brain. A study by Hajek et al. presented at the 2013 meeting of the International Society of Bipolar Disorders examined patients with bipolar disorder who had either received lithium for at least two years (37 patients) or had received under three months of treatment with lithium (19 patients), and compared the size of the hippocampus in these two groups and one control group (50 people). The patients with bipolar disorder all had the disorder for at least 10 years (25 years on average) and had had a minimum of five episodes.
Those treated with lithium long-term had greater hippocampal volume than the non-lithium patients (despite having spent more time in episodes of illness), and equal volume to healthy controls. Measurements were collected via magnetic resonance imaging (MRI), and analyses were done two different ways to avoid being confounded by the changes lithium may have on water balance in the brain, a phenomenon that was recently found to affect MRI images.
Editor’s Note: These data add to the large number of studies in animals and humans indicating that lithium, in addition to preventing episodes and suicides, may have neurotrophic and neuroprotective effects.
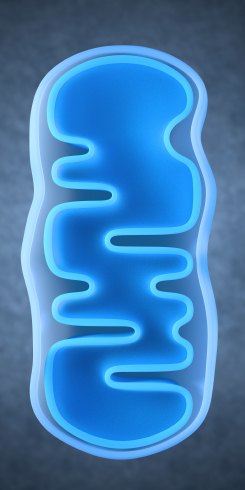
Lithium Reverses Effects of Oxidative Stress on Mitochondrial Function
Oxidative stress has been implicated in a wide range of illnesses, but what is it exactly? Our bodies use the oxygen we breathe to burn the fuel we get from food, and while this is a natural process, it produces byproducts known as free radicals, which are unstable molecules that can strip electrons from other molecules in a process called oxidation. Antioxidants (such as vitamin C) act as a source of electrons, helping keep other cells stable and healthy. Oxidative stress refers to the stress on our bodies from the normal effects of free radicals combined with environmental stressors like tobacco smoke or radiation.
In work presented at the 2013 meeting of the Society of Biological Psychiatry, Anna Andreason showed that over-activity of neurons increases oxidative stress through the production of reactive oxygen species (ROS). These are a type of free radicals that can damage cells in two ways: nitrosylation of proteins (adding nitric oxide to a thiol molecule), and oxidation, which results in more lasting effects on synaptic structures. The chemical compound rotenone damages mitochondria by producing ROS, and Andreason found that lithium was able to reverse this production and reverse the adverse effects of oxidative stress.
Lithium Has an Amazing Array of Positive Effects
Editor’s Note: The ability of lithium to protect mitochondria (the energy storehouse of a cell) adds to an increasingly long list of lithium’s neurotropic and neuroprotective benefits. Lithium increases cell survival factors BDNF and Bcl-2, increases markers of neuronal integrity such as N-Acetylaspartic acid (NAA), increases the volume of the hippocampus and cortex, and now helps protect mitochondria from oxidative stress. Lithium also increases the length of telomeres, which cap the ends of chromosome and protect them from damage during the DNA replication that occurs each time a cell divides. Short telomeres are associated with many kinds of medical and psychiatric diseases, as well as shorter life spans. No wonder that in addition to preventing mania and depression it has other clinical benefits, such as preventing memory deterioration, medical mortality, and suicide.
Inflammatory Markers May Predict Antidepressant Response
There appears to be a link between inflammation and depression. In the journal Neuropsychopharmacology, Cattanes et al. reported in 2013 that compared to controls, depressed patients had significantly higher baseline levels of inflammatory cytokines, less glucocorticoid receptor function, less neuroplasticity, and fewer neuroprotective factors. Certain variables predicted response to treatment, others were seen only in responders, and still others changed in everyone with antidepressant treatment.
Higher baseline levels of inflammatory markers interleukin IB, macrophage inhibitory factor (MIF), and tumor necrosis factor TNF? were each associated with nonresponse to antidepressant treatment, and the three combined accounted for 50% of the variance in response—that is, they were the major predictor of whether a patient responded to treatment.
Levels of other factors changed in only those patients who responded well to antidepressants. The biggest changes were the normalization in levels of the neurotrophic factors BDNF and VEGF.
Several other markers normalized with antidepressant treatment regardless of whether the patients responded to treatment, and these included decreases in cytokines interleukin-IB and MIF and improved glucocorticoid receptor function.
The three different kinds of findings about these biomarkers were observed regardless of what type of antidepressant was used—SSRI versus tricyclic nortriptyline (which blocks norepinephrine reuptake).
Editor’s Note: This study replicates other studies in depression where signs of inflammation have been observed, including increases in inflammatory cytokines, decreases in glucocorticoid receptor function (needed to suppress high levels of the stress hormone cortisol) and lower levels of neuroplasticity and neuroprotection markers. This, however, is one of the first studies to show that levels of these markers at baseline may predict response to antidepressant treatment.
Also novel are the findings that while some high interleukin levels at baseline predicted antidepressant non-response, other ones normalized only in responders, and still others changed with treatment independent of whether the patients’ depression improved. These exciting findings require replication, but suggest the future possibility of personalized medicine, that is, choosing medications based on an individual biochemical marker profile. Eventually direct use of anti-inflammatory agents may be necessary in those with the highest levels of cytokines (predicting non-response to conventional antidepressant treatment). The same types of studies are needed in bipolar depression to determine the relationship between these inflammatory markers and treatment response.
Proven Treatments for Fibromyalgia and Chronic Fatigue Syndrome
At the 2012 meeting of the Collegium Internationale Neuro-Psychopharmacologicum (CINP), a symposium was held to discuss fibromyalgia and chronic fatigue syndrome, two illnesses that remain mysterious.
Fibromyalgia
Fibromyalgia is more common in women than in men and is characterized by aching all over, decreased sleep, stiffness upon waking, and most prominently, being tired all day, as well as a host of other symptoms including headache, dizziness, and gastrointestinal upset. Researcher Siegried Kasper suggested that treating fibromyalgia requires more than just medication. His approach is known as MESS, which stands for medication, exercise, sleep management, and stress management.
Medications to treat the illness include milnacipran (not available in the US), duloxetine (Cymbalta, a serotonin-norepinephrine reuptake inhibitor or SNRI), or pregabalin (Lyrica), and if tolerated, low doses of the tricyclic amitriptyline (Elavil).
According to Kasper, SSRIs and anti-inflammatory drugs don’t work, and benzodiazepines decrease the deepest phase of sleep (stage 4) and can exacerbate the syndrome.
Recommended exercise is moderate, graded (to a pulse of about 120, or at a level where the patient can still talk, but can’t sing), and should be done in the early morning rather than the late afternoon where it might interfere with sleep.
Good sleep hygiene is recommended, such as keeping the same sleep schedule every day and abstaining from caffeine (even in the morning).
Working on developing active coping strategies for stressors that are likely to occur is a good idea. Mindfulness and other meditative techniques may also be helpful. Joining a support group (that encourages exercise rather than discouraging it) was also recommended.
Chronic Fatigue Syndrome
At the CINP meeting researcher Simon Wessely discussed chronic fatigue syndrome (CFS), which has many overlaps with fibromyalgia. He reported that careful controlled study of more than 15,000 individuals has not indicated that the illness is associated with a viral infection. Just as many people with and without chronic fatigue syndrome were found to be infected with a virus.
However, like the myth that vaccines cause autism, the myth that chronic fatigue is associated with a virus remains popular despite the lack of evidence. A large randomized study validated Wessely’s treatment techniques, but he has continued to be vilified for the position that the illness is not virally based. The study showed that patients who participated in cognitive behavior therapy and graded exercise improved more than those who received conventional medical management.
Wessely thought the most important cognitive change to make was accepting that exercise is not harmful for patients with chronic fatigue syndrome, and is in fact helpful and therapeutic. Many older treatment approaches had advocated rest, rest, and more rest, or even “intensive rest.” However, Wessely indicated that this would be counter-productive, as the patient would lose muscle mass and cardiovascular conditioning, and would become even more tired and chronically fatigued.
Prazosin Treats PTSD Nightmares
Patients with PTSD often struggle with nightmares, but a treatment normally used for high blood pressure may also be able to prevent these sometimes horrific dreams. In a study that marks the third replication of this finding, Murray Raskind, a researcher from the University of Seattle, reported that prazosin was significantly better than placebo at selectively blocking nightmares in 77 Iraq war veterans with PTSD in a 15-week trial. (Interestingly, normal dreaming is uninterrupted.)
Editor’s Note: Prazosin is a noradrenergic alpha-1 receptor antagonist. Doses of this drug must be titrated upward slowly over a period of 6 weeks to avoid orthostatic hypotension (a sudden fall in blood pressure that occurs when a person stands up). Maximum doses achieved in this study were 5mg mid-morning and 20mg at night (although 10mg at night is often effective). This treatment, although not FDA-approved, is increasingly being used in veteran populations and other patients with PTSD.
Long-term Treatment Response in Bipolar Illness
Willem Nolen, a researcher who has spent 40 years studying unipolar and bipolar disorder, recently retired from his position at Groningen Hospital in the Netherlands. In February, his retirement was celebrated with a symposium where he and other researchers discussed some of their important findings from the last several decades.
Nolen recently published a double-blind randomized study showing that in patients who were initially responsive to monotherapy with quetiapine (Seroquel), continuing the drug (at doses of 300-800mg/night) or switching to lithium were both more effective than switching to placebo over 72 weeks of long-term follow-up.
This study shows that quetiapine, which is only FDA-approved for long-term preventative treatment when used in combination with lithium or valproate (Depakote), also has efficacy when used as monotherapy.
Lithium is Highly Effective in Long-term Prevention
Nolen’s work also adds to an impressive amount of literature showing that lithium is highly effective in long-term prevention. This case is especially noteworthy because lithium was effective even in patients who had initially been selected for their response to quetiapine. (Studies that use this kind of “enriched sample” can only claim that quetiapine has long-term efficacy in those patients who initially respond well to the drug.) The data on lithium are even more impressive since the patients in this study were not enriched for lithium response.
Nolen has also conducted multiple studies of lithium, but optimal doses and target blood levels of the drug remain controversial. The therapeutic range of lithium is usually considered to be 0.6 to 1.2 meq/L, but some have argued that lower levels may still be effective. In a new analysis of those patients in the quetiapine study who were switched to lithium treatment, Nolen found that only lithium levels above 0.6 meq/L produced better results than placebo in long-term prophylaxis. Read more
ECT Update: Some good news and some not-so-good news
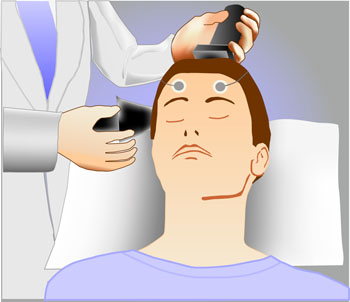 A 2013 study by Prudic et al. in the Journal of Electro-convulsive Therapy reveals some good and some not-so-good news about ECT. The good news about ECT is that it produced moderate acute remission rates. In this randomized study of ECT treatment, improvement rates were better when patients received right unilateral (RUL) ultra-brief pulse at high doses (6 times a patient’s seizure threshold) than with bilateral (BL) pulse at low doses (1.5 times the patient’s seizure threshold). RUL also has fewer cognitive side effects than BL.
A 2013 study by Prudic et al. in the Journal of Electro-convulsive Therapy reveals some good and some not-so-good news about ECT. The good news about ECT is that it produced moderate acute remission rates. In this randomized study of ECT treatment, improvement rates were better when patients received right unilateral (RUL) ultra-brief pulse at high doses (6 times a patient’s seizure threshold) than with bilateral (BL) pulse at low doses (1.5 times the patient’s seizure threshold). RUL also has fewer cognitive side effects than BL.
Prudic also found that these acute remission rates were best when antidepressant treatment was begun at the same time as ECT rather than after the end of ECT treatment.
Unfortunately, a previous study by Prudic et al. showed that relapse rates after ECT remain high. Two-thirds of patients relapse in the first six months after ECT. Half of patients who receive antidepressant treatment following ECT relapse within the first six months after their last ECT treatment. Twenty to forty percent relapse in the first month after their last ECT treatment.
In the current study, timing and likelihood of relapse was independent of whether antidepressant treatment was started at the outset of ECT or after the end of ECT. Relapse also did not depend on which pharmacological treatments are used (nortriptyine plus lithium versus venlafaxine plus lithium).
Older patients (average age 55) did better—they relapsed less often than patients with an average age of 45. Patients with unipolar and bipolar depression did not differ in relapse rates.
Previous history of illness did affect relapse. The number of prior antidepressant trials a patient had tried for a current depressive episode (a measure of treatment resistance) was related to how fast they relapsed on follow-up pharmacotherapy after receiving ECT (more antidepressant trials was associated with faster relapse).
Other studies have shown that continuation of ECT treatment is not superior to continued treatment with drugs following ECT treatment.
Editor’s Note: ECT works acutely, but too often its effects do not last long, even with intensive continuation treatment with an antidepressant and lithium. Therefore for patients with highly recurrent illness, its usefulness is largely limited to acute emergencies, such as high risk of suicide or medical deterioration.
There are currently no good controlled studies showing how to prevent depressive relapse after remission with ECT using either drug continuation therapy or maintenance ECT. Greater degrees of treatment resistance are associated with lower rates of both acute remission and faster relapse during follow-up pharmacotherapy.
If a patient is going to have ECT, RUL would be recommended over bilateral, because bilateral ECT is associated with decreases in autobiographical memory even after six months, and these deficits are in proportion to the number of bilateral ECT treatments received.
Alternatives to ECT
Other types of brain stimulation treatments could potentially serve as alternatives to ECT. Read more