Clinical Vignettes from Dr. Elizabeth Stuller
 Dr. Elizabeth Stuller, a staff psychiatrist at the Amen clinics in Washington, DC and CEO of private practice Stuller Resettings in Baltimore, MD, provided this editor (Robert M. Post) with several interesting anecdotal observations based on her wide clinical experience with difficult-to-treat mood disordered patients.
Dr. Elizabeth Stuller, a staff psychiatrist at the Amen clinics in Washington, DC and CEO of private practice Stuller Resettings in Baltimore, MD, provided this editor (Robert M. Post) with several interesting anecdotal observations based on her wide clinical experience with difficult-to-treat mood disordered patients.
- Stuller has used low-dose asenapine (Saphris), e.g. half a pill placed under the tongue, for depressed patients with alcohol use problems who have trouble getting to sleep. She has also used asenapine for rapid calming of agitated patients in her office.
- Stuller has also had success with the use of the atypical antipsychotic drug brexpiprazole (Rexulti) for patients with bipolar depression and low energy. She typically uses 0.5 mg/day for women and 1 mg/day for men. Stuller finds that there is little weight gain or akathisia with brexpiprazole.
- She has had success with the drug Nuedexta, which is a combination of dextromethorphan and quinidine and is approved for the treatment of sudden uncontrollable bouts of laughing or crying, known as pseudobulbar affect, which can occur as a result of neurological conditions or brain injuries. It is a combination of an NMDA antagonist and a sigma receptor agonist. Stuller starts with the 20mg dextromethorphan/10 mg quinidine dose once a day and increases to twice a day in week two. She finds it useful for behavioral effects of traumatic brain injury (TBI), anxiety resulting from the use of synthetic marijuana (sometimes called spice), and psychosis not otherwise specified. Stuller also finds that some patients appear to respond well to Nuedextra but not minocycline, or vice versa.
Editor’s Note: Note that these are preliminary clinical anecdotes conveyed in a personal communication, and have not been studied in clinical trials, thus should not be relied upon in the making of medical decisions. All decisions about treatment are the responsibility of a treating physician.
An Overview of Ketamine for Treatment-Resistant Depression
 A 2017 series of articles by researcher Chittaranjan Andrade in the Journal of Clinical Psychiatry reviews the last 10 years of research on ketamine, the anesthetic drug that in smaller doses (0.5 mg/kg of body weight) can bring about rapid antidepressant effects. Ketamine is typically delivered intravenously (though it can also be delivered via inhaler, injected under the skin or into muscles, and least effectively by mouth). Ketamine can improve depression in less than an hour, but its effects usually fade within 3 to 5 days. Repeating infusions every few days can extend ketamine’s efficacy for weeks or months.
A 2017 series of articles by researcher Chittaranjan Andrade in the Journal of Clinical Psychiatry reviews the last 10 years of research on ketamine, the anesthetic drug that in smaller doses (0.5 mg/kg of body weight) can bring about rapid antidepressant effects. Ketamine is typically delivered intravenously (though it can also be delivered via inhaler, injected under the skin or into muscles, and least effectively by mouth). Ketamine can improve depression in less than an hour, but its effects usually fade within 3 to 5 days. Repeating infusions every few days can extend ketamine’s efficacy for weeks or months.
Andrade cited a 2016 meta-analysis of nine ketamine studies by T. Kishimoto and colleagues in the journal Psychological Research. The meta-analysis found that compared to placebo, ketamine improved depression beginning 40 minutes after IV administration. Its effects peaked at day 1 and were gone 10–12 days later. Remission rates were better than placebo starting after 80 minutes and lasting 3–5 days.
Several studies have found that ketamine also reduces suicidality.
Andrade reported that both effectiveness and side effects seem to be dose-dependent within a range from 0.1 mg/kg to 0.75 mg/kg.
Side effects of ketamine are typically mild and transient. A 2015 study by Le-Ben Wan and colleagues (also in the Journal of Clinical Psychiatry) that Andrade cited reported that in 205 sessions of ketamine administration, the most common side effects were drowsiness, dizziness, poor coordination, blurred vision, and feelings of strangeness or unreality. The feelings of unreality (dissociative effects) diminish with repeated infusions. Heart and blood pressure may also temporarily increase as a result of ketamine administration.
One study found that ketamine could speed up and add to the effects of the selective serotonin reuptake inhibitor (SSRI) antidepressant escitalopram (Lexapro). A meta-analysis of 10 randomized controlled trials found that ketamine did not improve the effects of electroconvulsive therapy.
Ketamine has some history as a recreational club drug (sometimes known as ‘K’ or ‘special K’), and can be misused or abused.
While there have been many studies of ketamine’s antidepressant effects, Andrade concludes that none is of a standard to justify US Food and Drug Administration approval for the drug. It is hoped that larger, more rigorous trials will be completed in the next few years. However, ketamine is already being used widely to treat treatment-resistant unipolar and bipolar depression.
Deep TMS May Improve Treatment-Resistant Bipolar Depression
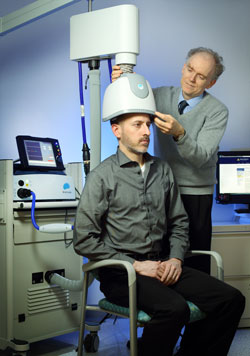 Deep transcranial magnetic stimulation (dTMS) is a non-invasive treatment that has been shown to be effective in unipolar depression. It consists of a helmet fitted to the head, which uses magnetic coils to create an electric field in a desired brain region.
Deep transcranial magnetic stimulation (dTMS) is a non-invasive treatment that has been shown to be effective in unipolar depression. It consists of a helmet fitted to the head, which uses magnetic coils to create an electric field in a desired brain region.
A 2017 double-blind randomized study by Diego F. Taveres and colleagues in the journal Neuropsychopharmacology found that 20 sessions of dTMS targeting the left dorsolateral prefrontal cortex produced greater improvement in bipolar depression over 4 weeks of treatment than the same number of sham sessions in which participants wore a helmet that delivered similar sounds and scalp sensations without the electrical effects to the brain. The participants had treatment-resistant bipolar depression that was being treated with medication.
However, dTMS’ effects were not significantly different from those of the sham over four additional weeks of follow-up, nor were remission rates significantly different across the two groups. Out of 50 participants, seven dropped out of the study—two from the sham group, and five from the active dTMS group. But there were no occasions on which a participant switched into mania following treatment.
This study suggests that dTMS has the potential to more rapidly improve treatment-resistant bipolar depression as well as unipolar depression.
Psilocybin May Improve Treatment-Resistant Depression
A small, uncontrolled study in the journal Lancet Psychiatry suggests that psilocybin, an ingredient in hallucinogenic mushrooms, relieved depression symptoms for up to three months in seven of 12 participants with unipolar depression that had not responded to at least two antidepressant medications.
Psilocybin has a different mechanism of action than typical treatments for depression. It activates 5HT2A serotonin receptors.
The participants, who had moderate to severe depression, were given two oral doses of psilocybin, a low dose (10mg) to establish the safety of the intervention, and a higher dose (25mg) seven days later. Psychedelic effects (anxiety, confusion, nausea, and headache) peaked within two to three hours and had dissipated by six hours after the intervention.
Depression began to improve within 24 hours after the 25mg dose. Depression symptoms were significantly improved by one week after the intervention. Eight of the 12 participants had a complete remission of their depression after one week, and this lasted the full three months in five participants. By the end of the three months, a total of seven of the 12 participants met the criteria for response to psilocybin.
The study’s authors, led by Robin L. Carhart-Harris, suggest that their preliminary results warrant more systematic investigation of psilocybin, but because there was no comparison group in this study, a large placebo effect cannot be ruled out.
Efficacy of Direct Current Stimulation in Major Depression
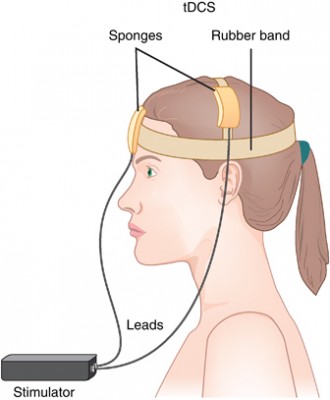 A new meta-analysis presented at the 2015 meeting of the Society of Biological Psychiatry has clarified the efficacy of transcranial direct current stimulation (tDCS) in major depression. TDCS is a treatment in which electrodes deliver a steady low level of electrical stimulation to the brain. The meta-analysis presented by Andre Brunoni and colleagues used individual patient data from six recent studies comparing tDCS treatment to a sham treatment, totaling 289 patients. TDCS treatment was superior to the sham control in terms of antidepressant response (34% to 19%), remission rates (23.1% to 12.7%), and improvement in depression.
A new meta-analysis presented at the 2015 meeting of the Society of Biological Psychiatry has clarified the efficacy of transcranial direct current stimulation (tDCS) in major depression. TDCS is a treatment in which electrodes deliver a steady low level of electrical stimulation to the brain. The meta-analysis presented by Andre Brunoni and colleagues used individual patient data from six recent studies comparing tDCS treatment to a sham treatment, totaling 289 patients. TDCS treatment was superior to the sham control in terms of antidepressant response (34% to 19%), remission rates (23.1% to 12.7%), and improvement in depression.
After adjusting for confounding factors, the researchers found that patients who had failed to respond to previous treatments were less likely to respond well to tDCS than other patients. They also found that higher doses of tDCS (in terms of current density, duration, and number of sessions) predicted a better response than lower doses.
Over 5 Years, Vagal Nerve Stimulation Better than Treatment as Usual for Severe Depression
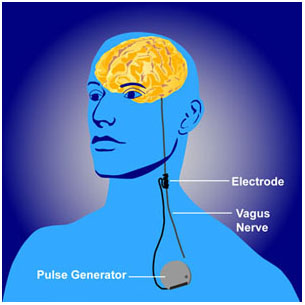 Vagal nerve stimulation (VNS) is an FDA-approved treatment for both seizures and depression that has resisted other treatments. It requires an operation for the insertion of a stimulator in the chest wall and electrodes on the left vagus nerve in the neck. A new study by Scott T. Aaronson and colleagues presented at the 2015 meeting of the Society of Biological Psychiatry observed severely depressed patients, 494 who received VNS and 301 who received treatment as usual in the community, over a period of five years. The patients who received VNS had greater response rates, they were more likely to have experienced remission, and their remissions lasted longer than those who received treatment as usual. Overall the patients who received VNS had lower mortality rates and suicide rates as well. VNS might be a good option for patients with depression that has not responded to most other treatments.
Vagal nerve stimulation (VNS) is an FDA-approved treatment for both seizures and depression that has resisted other treatments. It requires an operation for the insertion of a stimulator in the chest wall and electrodes on the left vagus nerve in the neck. A new study by Scott T. Aaronson and colleagues presented at the 2015 meeting of the Society of Biological Psychiatry observed severely depressed patients, 494 who received VNS and 301 who received treatment as usual in the community, over a period of five years. The patients who received VNS had greater response rates, they were more likely to have experienced remission, and their remissions lasted longer than those who received treatment as usual. Overall the patients who received VNS had lower mortality rates and suicide rates as well. VNS might be a good option for patients with depression that has not responded to most other treatments.
Meta-Analysis Shows Effectiveness of Ketamine for Bipolar and Unipolar Depression
Ketamine, an anesthetic sometimes used intravenously in the treatment of depression, can bring about rapid onset of antidepressant effects. A new meta-analysis by researcher Michael Bloch and colleagues presented at a recent conference showed that ketamine’s maximum antidepressant effects occur within one day of administration, and its effects remain significant (compared to control conditions) one week following infusion. Ketamine’s effects were diminished in patients taking other medications. There was a trend for better response in patients with bipolar disorder than with unipolar disorder.
Bloch and colleagues analyzed eight earlier studies including a total of 180 participants. In each study, ketamine had been compared to a control condition, either an infusion of saline solution or of midazolam, which mimics ketamine’s sensory effects but does not have antidepressant effects. The researchers are calling for more meta-analyses of ketamine studies to determine which patients respond best to ketamine and how to sustain ketamine’s effects.
Editor’s Note: In another poster presented at the same conference, James Murrough reported that patients with slower processing speed responded best to ketamine. Other findings have shown that those with a history of alcohol abuse and a common genetic variant of brain-derived neurotrophic factor (BDNF), the val-66-val allele of proBDNF, are more likely to respond to ketamine.
ECT versus Drug Therapy for Bipolar Depression
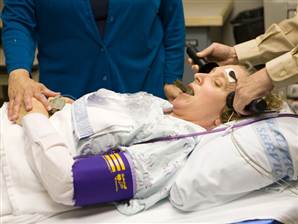 Electroconvulsive therapy is often considered a primary treatment option for patients with severe bipolar disorder that has resisted pharmacological treatment. Researcher Helle K. Schoeyen and colleagues recently published the first randomized controlled trial comparing ECT (in this case right unilateral brief pulse ECT) with algorithm-based pharmacological treatment in 76 patients with treatment-resistant bipolar depression.
Electroconvulsive therapy is often considered a primary treatment option for patients with severe bipolar disorder that has resisted pharmacological treatment. Researcher Helle K. Schoeyen and colleagues recently published the first randomized controlled trial comparing ECT (in this case right unilateral brief pulse ECT) with algorithm-based pharmacological treatment in 76 patients with treatment-resistant bipolar depression.
The response rate was significantly higher in the ECT group than in the patients who received drug treatment (73.9% versus 35.0%). However, the two treatment groups had similarly low remission rates (34.8% for ECT and 30.0% for pharmacological treatment).
The algorithm-based pharmacological treatment used in the study was based on a sequence of treatments endorsed by researchers Frederick K. Goodwin and Kay Redfield Jamison in their 2007 book Manic-Depressive Illness. A selected treatment was chosen for each participant based on his or her medical history. If the first treatment was ineffective or intolerable, the patient would be switched to the next treatment option. Antipsychotics, antidepressants, anxiety-reducing drugs, and hypnotics were some of the other treatments included in the algorithms.
Patients in the study had previously showed a lack of response to at least two different antidepressants and/or mood stabilizers with documented efficacy in bipolar disorder (lithium, lamotrigine, quetiapine, or olanzapine) in adequate doses for a period of 6 weeks (or until they quit because of side effects).
Editor’s Note: Even when ECT is effective, there is the issue of how to maintain that good response. We previously reported that in a 2013 study by Axel Nordenskjöld et al. in the Journal of ECT, intensive followup treatment with right unilateral brief pulse ECT combined with pharmacotherapy was more effective than pharmacology alone at preventing relapses. Patients who improved after an acute series of ECT (three times/week) then received weekly ECT for six weeks and every two weeks thereafter, totaling 29 ECT treatments in one year.
Other studies of more intermittent continuation ECT have not proved more effective than medication. Thus high intensity right unilateral brief pulse ECT is one option for extending the effects of successful ECT.
More Data on Memantine for Treatment-Resistant Depression
In 2012 we reported on an open study by Athanasios Koukopoulos and colleagues that explored whether the NMDA glutamate receptor antagonist memantine (Namenda), which is used to treat dementia, could be helpful to people with treatment-resistant bipolar disorder. In an update of that study, the researchers, led by Giulia Serra, compared patients’ symptoms during three years of treatment as usual, followed by three years with memantine added to their stable medication regime (at doses of 20–30 mg/day). Patients improved progressively over the three years of taking memantine.
Improvements in symptoms included decreased time ill, decreased severity of symptoms, decreased duration of new episodes, and fewer episodes per year. Memantine was particularly helpful for those patients who had had rapid or continuous cycling. Side effects were minimal.
Given the success of this open study, randomized controlled trials are needed to explore this much-needed option for people with treatment-resistant bipolar disorder.
New Antidepressant Vortioxetine May Improve Cognition and Treatment-Resistant Depression
Vortioxetine (Brintellix) is a new antidepressant that has a range of effects on serotonin receptors, making it different from selective serotonin reuptake inhibitors (SSRIs), the most common type of antidepressants, which work only on the serotonin transporter. Researcher Johan Areberg et al. reported at the 2014 meeting of the American Psychiatric Association that the drug is an antagonist at receptors 5-HT3, 5-HT7, and 5-HT1D; a partial agonist at 5-HT1B; a full agonist at 5-HT1A; and an inhibitor of the 5-HT transporter. The researchers suggested that at doses of 5mg/day, vortioxetine occupies the 5-HT3 receptors and 50% of the serotonin transporter. As dosage increases to 20mg/day, vortioxetine is believed to occupy all of the serotonin targets at clinically relevant levels. Doses of 20mg/day were found to be effective in nine studies. Researcher Gennady Smagin et al. also reported that vortioxetine activates central histamine receptors.
Vortioxetine appears to be useful in patients who have previously failed to respond to antidepressants. Researcher George I. Papakostas et al. reported that in a cohort of about 500 patients who responded inadequately to previous prescriptions of selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs), the 252 taking vortioxetine improved more than the 241 taking the antidepressant agomelatine.
Editor’s Note: Vortioxetine’s superior effects are impressive, as agomelatine, which is approved for use in at least 41 countries including the UK, Canada, and Australia, but is not available in the US, has previously been shown to be more effective than a number of SSRIs in head-to-head comparisons. Agomelatine improves sleep and circadian rhythms via its dual effects as an agonist at melatonin M1 and M2 receptors and an inhibitor of 5HT2C receptors, which results in the release of norepinephrine and dopamine in the frontal cortex.
Vortioxetine may be unique among antidepressants in that it appears to improve cognition. Researcher John E. Harrison et al. reported that patients saw increases in executive function, attention, speed of processing, and memory while taking vortioxetine. This is consistent with studies in aged mice, whose cognition improves more on vortioxetine than on the SSRI fluoxetine, according to researcher Yan Li and colleagues.