Newly Identified Effects of N-Acetylcysteine
 In a talk at the 2019 meeting of the International Society for Bipolar Disorders, researcher Michael Berk, who was responsible for some of the initial findings on the effects of the antioxidant N-acetylcysteine (NAC), summarized some of the newer findings about the treatment.
In a talk at the 2019 meeting of the International Society for Bipolar Disorders, researcher Michael Berk, who was responsible for some of the initial findings on the effects of the antioxidant N-acetylcysteine (NAC), summarized some of the newer findings about the treatment.
NAC has been found to be effective in bipolar depression and in the treatment of both positive and negative symptoms of schizophrenia. It also helps in the avoidance of cocaine, alcohol, tobacco, and marijuana. It can reduce habitual behaviors such as gambling, obsessive compulsive disorder (OCD), and trichotillomania (compulsive hair-pulling) and irritability and motor stereotypy (repeated movements) in autism.
A 2016 study by researcher Sudie E. Back and colleagues in the Journal of Clinical Psychiatry found that NAC improved symptoms of post-traumatic stress disorder (PTSD) in veterans who also had depression and substance use disorders at a dosage of 2.4 grams/day.
According to Berk, NAC also reduces the incidence of lithium-related renal failure and reduces mitochrondrial toxicity. One study reported that it improved working memory in patients with schizophrenia.
In his talk, Berk also noted that statins offer an interesting new avenue for treatment. Several studies have suggested statins can improve mood or reduce the likelihood of a depressive recurrence. Angiotension-active drugs (inhibitors) have also been reported to decrease the incidence of depression and to improve cognition.
Meta-Analysis Finds Omega-3 Fatty Acids Do Not Reduce Cardiovascular Disease Risk
 In a 2018 meta-analysis published in the journal JAMA Cardiology, researcher Theingi Aung and colleagues found that across 10 studies including a total of 77,197 participants, omega-3 fatty acid supplementation did not reduce risk of coronary heart disease in people at high risk. This newer finding conflicts with a 2017 advisory from the American Heart Association that suggested omega-3 fatty acid supplementation might prevent cardiovascular disease.
In a 2018 meta-analysis published in the journal JAMA Cardiology, researcher Theingi Aung and colleagues found that across 10 studies including a total of 77,197 participants, omega-3 fatty acid supplementation did not reduce risk of coronary heart disease in people at high risk. This newer finding conflicts with a 2017 advisory from the American Heart Association that suggested omega-3 fatty acid supplementation might prevent cardiovascular disease.
When it comes to mood disorders, it has been similarly difficult to pin down whether omega-3 fatty acids are helpful. Data on omega-3 fatty acid supplements for the prevention of depression have been ambiguous, with small numbers of studies and variations in study design that make it difficult to draw strong conclusions about whether these supplements can improve or prevent depression.
A 2016 systematic review by Paola Bozzatello and colleagues in the Journal of Clinical Psychiatry found only seven studies of omega-3 fatty acid supplementation in bipolar disorder. The studies had small sample sizes and widely varying dosage parameters, so the evidence that can be drawn from them is not strong, but the review did find a modest benefit on bipolar depression (but not mania) when omega-3 fatty acids were added to a treatment regimen, compared to treatment as usual.
The same review found that studies of omega-3 fatty acid supplementation in unipolar depression also varied widely, and thus it was difficult to draw inferences from them. Some meta-analyses found no benefit to omega-3 fatty acid supplementation, while others suggested that omega-3s could improve depression. The review found that the type of omega-3 fatty acids used might matter. Supplementation with EPA seemed to improve depression more than supplementation with DHA. The review also cited a 2014 comprehensive meta-analysis by Giuseppe Grosso and colleagues in the journal PLoS One that analyzed the findings from 19 studies in people with depression or depressive symptoms. Grosso and colleagues found that people with more severe depression seemed to benefit more from omega-3s.
Antioxidant Supplement Coenzyme Q10 Looks Promising for Bipolar Depression
Coenzyme Q10 (CoQ10) is an antioxidant that occurs naturally in the human body, but its levels decline with age, medical illness, and depression. In a randomized, controlled trial that was published in the Journal of Clinical Psychopharmacology in 2018, researcher Maryam Mehrpooya and colleagues found that adding coenzyme Q10 supplements to a treatment regimen improved bipolar depression compared to adding placebo.
The pathophysiology of bipolar disorder involves mitochondrial dysfunction, oxidative stress, and inflammation, and coenzyme Q10 can affect all of these pathways. It is also neuroprotective, and may help prevent the degeneration of neurons in people with Alzheimer’s, Parkinson’s, or Huntington’s diseases.
The study included a final total of 69 participants who were randomly assigned to receive either 200 mg/day of coenzyme Q10 supplements or placebo in addition to their normal treatment regimen, which had been stable for at least two months at the time of the study. Participants’ bipolar depression was rated at the beginning of the study, after four weeks, and after eight weeks. At the eight-week mark, coenzyme Q10 showed a statistically significant benefit over placebo with a large effect size. Three participants who received coenzyme Q10 experienced full remission of their depression, and 72% of those in the coenzyme Q10 group improved compared to only 12% of those who received placebo.
The study had some limitations. It was small, and twenty participants dropped out of the study before its completion, which may have inflated the findings.
Previous research found that coenzyme Q10 had benefits in specific populations. In two non-blind studies (studies in which participants know that they are receiving the treatment in question rather than possibly a placebo), 29 older patients with bipolar disorder improved when taking 800 mg to 1200 mg/day of coenzyme Q10. A randomized, controlled trial of coenzyme Q10 in people with multiple sclerosis and depression found that 500 mg/day reduced fatigue symptoms and depression. Coenzyme Q10 has also improved well-being and energy in small, controlled trials in people with breast cancer, Gulf War veterans, and elderly populations.
Taking coenzyme Q10 is low-risk. It had no adverse effects in the study by Mehrpooya and colleagues. Gastrointestinal reactions are possible, but can be managed by taking coenzyme Q10 with food and spreading out dosing throughout the day. Insomnia is also possible, but is less likely when coenzyme Q10 is taken early in the day. One effect to note is that coenzyme Q10 can interact badly with the blood-thinner warfarin.
Editor’s Note: The study by Mehrpooya and colleagues is interesting. Another antioxidant, N-acetylcysteine (NAC), also took 2 months to work in trichotillomania and bipolar depression, so patients should be warned not to expect a quick response with either coenzyme Q10 or NAC. Other potentially useful supplements include: Vitamin D3 (1500–5000 IU/day), folate or L-methylfolate, and acetyl-L-carnitine. Acetyl-L-carnitine may work more quickly, based on its presumed mechanism (increasing the production of the inhibitory metabotrophic glutamate receptor mGluR-2, which inhibits glutamate release).
Risk Gene for Bipolar Disorder Implicated in Depressed Behaviors and Abnormal Firing of GABA Neurons
At a 2018 scientific meeting and in a 2017 article in the journal PNAS, researcher Shanshan Zhu and colleagues reported that mice genetically engineered to lack the protein Ankyrin-G in certain neurons showed increases in depression- and mania-like behavior after being exposed to defeat stress (by repeatedly being placed in physical proximity to a larger, more aggressive mouse), which is often used to model human depression.
The researchers targeted the gene ANK3, which is responsible for the production of Ankyrin-G, and has been linked to bipolar disorder in genome-wide association studies. By manipulating the gene, they could eliminate Ankyrin-G in pyramidal neurons in the forebrain, a region relevant to many psychiatric disorders. Pyramidal neurons perform key brain functions, sending nerve pulses that lead to movement and cognition.
The missing Ankyrin-G affected sodium channels (which allow for the flow of sodium ions in and out of cells) and potassium channels. The neurochemical GABA (which typically inhibits nerve impulses) was also dysregulated, resulting in the kind of disinhibition seen in psychosis. Mice showed dramatic behavioral changes ranging from hyperactivity to depression-like behavior (e.g. giving up in a forced swimming test). The hyperactivity decreased when the mice were given treatments for human mania, lithium or valproic acid.
While mutations in the ANK3 gene may disturb sodium channels, another gene linked to depression and bipolar disorder, CACNA1C, affects calcium channels.
In a related study by researcher Rene Caballero-Florán and colleagues that was also presented at the meeting, mice were genetically engineered in such a way that interactions between Ankyrin-G and GABA Type A Receptor-Associated Protein (GABARAP) were disrupted, leading to deficits in inhibitory signaling. These deficits were partially corrected when the mice were treated with lithium.
The study by Caballero-Florán and colleagues used mice with a mutation known as W1989R in the ANK3 gene. Through a program that examines the genes of people with bipolar disorder, the researchers also identified a family with this genetic mutation, including a patient with type I bipolar disorder with recurrent mania and depression who has responded well to lithium treatment.
Lithium Superior to Other Mood Stabilizers in a Longitudinal Study of Bipolar Youth
At a late-2018 scientific meeting, researcher Danella Hafeman and colleagues reported some results of the Course and Outcome of Bipolar Youth (COBY) study. The study includes long-term follow up of 413 youth with bipolar disorder, who ranged in age from 7 to 17 years old. Hafeman and colleagues reported that taking lithium more than 75% of the time was linked to fewer suicide attempts, fewer depressive symptoms, and fewer psychosocial difficulties than taking another mood stabilizer (such as an atypical antipsychotic, lamotrigine, or valproic acid) more than 75% of the time after adjusting for demographic variables.
Despite the limitations of observational studies such as this one, the authors concluded, “Our findings are consistent with studies in adult populations, showing that lithium (compared to other mood stabilizers) is associated with decreased suicidality, less depression, and better psychosocial functioning. Given the paucity of evidence regarding lithium in children and adolescents, these findings have important clinical implications for the pharmacological management of youth with [bipolar disorder].”
Editor’s Note: These observations are consistent with several other studies. Researcher Barbara Geller and colleagues observed in eight years of follow up of children diagnosed with bipolar disorder that those who were treated with lithium spent more time in remission than those who took other medicines. A randomized controlled study by researcher Robert Findling and colleagues documented that maintenance lithium treatment was more effective than placebo at preventing bipolar episodes. Together, these data suggest that lithium should be used more often in the long-term treatment of children with bipolar disorder.
Way ahead of his time in about 1993, the renowned child psychiatrist Dennis Cantwell said something like this: “If I had an adolescent child with a first manic episode, I would have him stay on lithium for the rest of his life.” He seems to have been prescient, as evidence of the many benefits of lithium over other alternatives in the treatment of both children and adults has been accumulating.
An open-access review article this editor (Robert M. Post) published in the journal Neuropsychopharmacology in 2017, “The New News about Lithium: An Underutilized Treatment in the United States,” argues that lithium’s many benefits have been underestimated, while its side effects have been overestimated. It is my view that it would be beneficial if lithium were more often included in the treatment regimen of adults as well as children and adolescents with bipolar disorder.
Lithium has an astounding range of effectiveness. It prevents recurrent depressions and suicide (even in those with unipolar depression), increases hippocampal and cortical volume, protects memory, and increases the length of telomeres (the end portions of chromosomes that protect them as they replicate). In multiple animal models of neurological diseases, it has also been found to be neuroprotective and to reduce the size of brain lesions.
Using Light to Improve Sleep and Depression
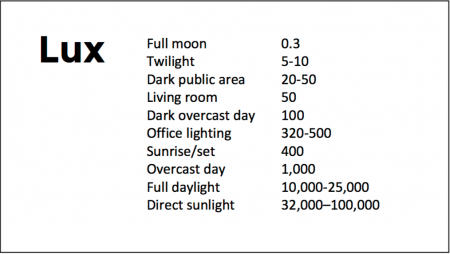
At the 2018 meeting of the North Carolina Psychiatric Association, researcher Chris Aiken described the phenomenon of sleep inertia, when people are awakened from deep sleep by an alarm, rather than waking at the end of a sleep cycle, and are groggy for 15 minutes. Depressed people may stay groggy for 4 hours. A dawn simulator may help. These lights turn on gradually over the course of 30 to 60 minutes, reaching 250 lux while the patient is still asleep. Dawn simulators have worked in eight out of ten controlled clinical trials to help people with seasonal affective disorder, adolescents, and normal adults wake up more easily. They range in cost from $25 to $90 and some brands include PER2LED or LightenUp. Aiken says dawn simulators can improve depression, sleep quality, and cognition.
Evening and nighttime light: Bright lights and blue light, like the light that comes from electronic screens, can shut down the body’s secretion of melatonin, making people awake and alert in the evening when they should be getting sleepy. Dim light or glasses that filter out blue light allow increases in melatonin secretion in the evening, while bright light suppresses it. Missing this early melatonin pulse creates “night owls” who have delayed sleep onset.
Because light still reaches our eyes through our eyelids as we sleep, even low-level light during the night impairs sleep, cognition, and learning, and increases the risk of depression by a hazard ratio of 1.8 (about double the risk). A 2017 study by Kenji Obayashi in the American Journal of Epidemiology found that bedroom light above 5 lux elevated rates of depression in older adults after two years of followup. Living room light averaged around 50 lux and increased depression further.
The treatment is turning off TVs, electronic screens, and cellphones in the evening or wearing blue-blocking glasses, which can be found for less than $10. Blue-blocking glasses can increase calmness and reduce anxiety, and even are effective in treating mania. Then, during sleep, wear an eye mask or get light-blocking blinds or curtains for windows. For a complete blackout, use blackout curtains, aluminum foil over windows, electric tape over LED lights, or try sleeping in the basement.
Aiken suggests that to re-instate healthy sleep patterns, people should institute virtual darkness from 6pm to 8am, including wearing blue-blocking glasses when out of bed. Then they should institute total darkness or wear an eye mask when in bed. When symptoms improve, this routine can gradually be shifted to begin later in the evening, such as two hours before bedtime.
Blue light filters are also available for smartphones and tablets including Apple Nightshift mode, Kindle BlueShade, and Android Twilight and Blue Light Filter.
Glasses that filter out blue light include Uvex Ultraspec 2000, 50360X ($7 on Amazon) and Uvex Skyper 351933X ($7-10 on Amazon). The website lowbluelights.com sells blue-blocking glasses from $45 and a variety of other blue-free lighting products such as lightbulbs and flashlights.
Bright light therapy for unipolar and bipolar depression: 30 minutes of bright light (7,500 to 10,000 lux) in the morning can help treat depression in unipolar and bipolar disorder and seasonal affective disorder. The effects usually take 3 to 7 days to set in, but they only last while a patient continues using the bright light in the morning. Researcher Dorothy K. Sit and colleagues found that bright light therapy in the morning sometimes caused hypomanic reactions in people with bipolar disorder, and reported in a 2018 article in the American Journal of Psychiatry that midday light therapy (from noon to 2:30pm) was also effective without this unwanted effect. However, a 2018 article by Ne?e Yorguner Küpeli and colleagues in the journal Psychiatry Research suggested that a half hour of morning light for two weeks was sufficient to bring about improvement in 81% of patients with bipolar disorder and did not cause serious side effects.
Melatonin regimen for sleep onset delay: Melatonin can be used to treat severe night-owls with a very late onset of sleep (for example, going to bed at 2 or 3am and sleeping late into the morning). Melatonin can help with sleep onset to some extent when used at bedtime, but in those with an extreme phase shift, researcher Alfred J. Lewy recommends a regimen of low dose priming with 400–500 micrograms of melatonin at 4pm and then a full dose of 3 milligrams of melatonin at midnight. The 4pm priming dose helps pull back the delayed onset of the body’s secretion of melatonin toward a more normal schedule.
Third Study Suggests Cariprazine Is Effective in Bipolar Depression
The atypical antipsychotic drug cariprazine (sold under the name Vraylar in the US) is currently approved by the US Food and Drug Administration for the treatment of schizophrenia and manic or mixed episodes of bipolar disorder. Based on recent successful phase 3 trials in bipolar depression, the pharmaceutical companies that produce cariprazine, Allergan and Gedeon Richter, plan to apply for a change in FDA labeling later this year to reflect the drug’s apparent ability to treat bipolar depression as well.
While many drugs can prevent or treat mania, treating bipolar depression has typically been more of a challenge. The most recent 6-week trial of cariprazine in 493 patients showed that a dose 1.5mg/day was significantly more effective than placebo at reducing depression ratings. (A dose of 3mg/day did not show superiority over placebo as it had in previous trials of cariprazine.)
Side effects reported in the trial were mild and included restless legs, nausea, and fatigue. Five percent of those who received cariprazine discontinued the drug due to side effects, compared to three percent of those who received placebo.
The mechanism by which cariprazine improves depression is not yet clear. The drug is a dopamine partial agonist, but unlike aripiprazole (Abilify) and brexpiprazole (Rexulti), which have more potent effects on D2 receptors than on D3 receptors, cariprazine is more potent at dopamine D3 receptors. Whether this difference accounts for the positive effects in bipolar depression that aripiprazole and brexpiprazole do not have remains to be seen.
FDA Approves Lurasidone for Bipolar Depression in Children and Adolescents
 In March 2018, the US Food and Drug Administration approved the antipsychotic drug lurasidone (Latuda) for the treatment of bipolar depression in children and adolescents aged 10–17 years. Lurasidone was already approved for adults with bipolar depression, as an add-on treatment to the mood stabilizers lithium and valproate, and for schizophrenia in people aged 13 years and up.
In March 2018, the US Food and Drug Administration approved the antipsychotic drug lurasidone (Latuda) for the treatment of bipolar depression in children and adolescents aged 10–17 years. Lurasidone was already approved for adults with bipolar depression, as an add-on treatment to the mood stabilizers lithium and valproate, and for schizophrenia in people aged 13 years and up.
A 6-week clinical trial in 347 youth compared lurasidone (in doses ranging from 20 to 80 mg/day) to placebo and found that those who received lurasidone showed significant improvements in depression compared to those who received placebo. The average dose was below 40 mg/day. The research by Melissa P. DelBello and colleagues was published in the Journal of the American Academy of Child and Adolescent Psychiatry in 2017.
In the study, lurasidone was well-tolerated. Side effects included nausea, sleepiness, minimal weight gain, and insomnia. Lurasidone did not seem to affect glucose, triglycerides, cholesterol, or blood pressure.
Editor’s Note: This is the first drug to be approved for bipolar depression in this age range. This editor (Robert M. Post) has written extensively on the high incidence of childhood onset bipolar disorder in the US, and especially in the offspring of parents with bipolar disorder.
It is important to be alert to the possibilities of depression and bipolar disorder in children in the US (along with related illnesses such as anxiety, oppositional defiant disorder, and attention deficit hyperactivity disorder (ADHD)), as early-onset illness tends to have a more severe long-term course than adult-onset depression and bipolar disorder. A longer delay between the emergence of symptoms and the first treatment for bipolar disorder is also a risk factor for more severe depression, more time depressed, and a poorer outcome in adulthood.
Parents of children aged 2-12 who have mood or behavioral problems are encouraged to consider joining the Child Network at our website, bipolarnews.org (click on the tab for the Child Network). By participating in this research network, parents are able to make a weekly rating of the severity of their children’s symptoms of anxiety, depression, ADHD, oppositional behavior, and mania via the secure website. The ratings can then be shared with the child’s clinicians for easy visualization of the course of symptoms over time, which may help with treatment decisions.
Clinical Vignettes from Dr. Elizabeth Stuller
 Dr. Elizabeth Stuller, a staff psychiatrist at the Amen clinics in Washington, DC and CEO of private practice Stuller Resettings in Baltimore, MD, provided this editor (Robert M. Post) with several interesting anecdotal observations based on her wide clinical experience with difficult-to-treat mood disordered patients.
Dr. Elizabeth Stuller, a staff psychiatrist at the Amen clinics in Washington, DC and CEO of private practice Stuller Resettings in Baltimore, MD, provided this editor (Robert M. Post) with several interesting anecdotal observations based on her wide clinical experience with difficult-to-treat mood disordered patients.
- Stuller has used low-dose asenapine (Saphris), e.g. half a pill placed under the tongue, for depressed patients with alcohol use problems who have trouble getting to sleep. She has also used asenapine for rapid calming of agitated patients in her office.
- Stuller has also had success with the use of the atypical antipsychotic drug brexpiprazole (Rexulti) for patients with bipolar depression and low energy. She typically uses 0.5 mg/day for women and 1 mg/day for men. Stuller finds that there is little weight gain or akathisia with brexpiprazole.
- She has had success with the drug Nuedexta, which is a combination of dextromethorphan and quinidine and is approved for the treatment of sudden uncontrollable bouts of laughing or crying, known as pseudobulbar affect, which can occur as a result of neurological conditions or brain injuries. It is a combination of an NMDA antagonist and a sigma receptor agonist. Stuller starts with the 20mg dextromethorphan/10 mg quinidine dose once a day and increases to twice a day in week two. She finds it useful for behavioral effects of traumatic brain injury (TBI), anxiety resulting from the use of synthetic marijuana (sometimes called spice), and psychosis not otherwise specified. Stuller also finds that some patients appear to respond well to Nuedextra but not minocycline, or vice versa.
Editor’s Note: Note that these are preliminary clinical anecdotes conveyed in a personal communication, and have not been studied in clinical trials, thus should not be relied upon in the making of medical decisions. All decisions about treatment are the responsibility of a treating physician.
TDCS Effective in Bipolar Depression
 A 2017 study in the journal JAMA Psychiatry reports that transcranial direct current stimulation (tDCS) is an effective add-on treatment for bipolar depression. In the study by researcher Bernardo Sampaio-Junior and colleagues, 59 patients taking medication for bipolar disorder and experiencing a depressive episode were randomized to receive either 10 daily half-hour sessions of tDCS (and then one every two weeks) or an inactive sham stimulation.
A 2017 study in the journal JAMA Psychiatry reports that transcranial direct current stimulation (tDCS) is an effective add-on treatment for bipolar depression. In the study by researcher Bernardo Sampaio-Junior and colleagues, 59 patients taking medication for bipolar disorder and experiencing a depressive episode were randomized to receive either 10 daily half-hour sessions of tDCS (and then one every two weeks) or an inactive sham stimulation.
TDCS is a painless form of neurostimulation in which electrodes applied to the scalp provide a steady, low current of electricity that modulates neuron activity. Sampaio-Junior describes its low cost, portability and ease of use as some of its benefits. This is the first randomized, sham-controlled study of tDCS in bipolar disorder.
After six weeks of treatment, patients who received real tDCS treatment showed significantly more improvement in their depression than those who received the inactive sham stimulation. In the active group, 67.6% showed sustained response compared to 30.4% in the inactive group. TDCS was well tolerated, with skin redness at the application site the only side effect that was more common in the active group than in the sham group. Mood switching rates were similar across the two groups.
The research was completed as part of the Bipolar Depression Electrical Treatment Trial (BETTER) taking place in Brazil. The group of participants was 68% female with a mean age of 45.9 years. Sixty-one percent of participants had bipolar I disorder while the remainder had been diagnosed with bipolar II.