The Unfolding Story of Poor Response to Antidepressants in Bipolar Depression
The role of the traditional antidepressants in the treatment of depression in bipolar illness remains controversial. Despite mounting evidence that they are not efficacious in the treatment of bipolar depression, they are still among the most widely used treatments for that condition. At the first biennial conference of the International Society for Bipolar Disorders held in Istanbul this past March, Mark A. Frye and Shigenobu Kanba chaired a symposium on antidepressant-induced mania and individualized treatment for bipolar depression.
This editor (Robert M. Post) discussed factors influencing antidepressants’ effects on patients with bipolar depression. In a recent meta-analysis, researchers Sidor and MacQueen reviewed data from studies encompassing 2373 patients with bipolar depression and found that antidepressants had no significant benefits over placebo on measures of response or remission. Pooled estimates for a thousand patients showed no increase in patients’ risk of switching into mania after treating with antidepressants. However, in a smaller sub-analysis, the risks of switching into mania following treatment with the older tricyclic antidepressants (43%) and venlafaxine (15%) was greater than the risk of switching after being treated with SSRIs (7%) or bupropion (5%).
There is a conundrum in the literature. While antidepressants don’t work very well in bipolar depression, there is a small subgroup of patients who, having responded well to antidepressants for two months, benefit more from continuing the antidepressant treatment than from discontinuing the drug. Continued treatment with adjunctive antidepressants (added to regular treatment with a mood stabilizer or an atypical antipsychotic) was associated with fewer relapses into depression over the next year when the antidepressants were continued compared to when they were discontinued. Lori Altshuler et al. have published two uncontrolled studies to this effect, Russell Joffe et al. have published one, and a more recent randomized study of this by Nassir Ghaemi replicated some of the results in patients who had non-rapid-cycling bipolar disorder. At the same time, the literature shows that there are number of risk factors for switching into hypomania during antidepressant treatment in bipolar depression.
Risk factors for switching into mania upon treatment with an antidepressant include: younger age, bipolar I compared to bipolar II, rapid cycling in the past year, mixed depression, use of older tricyclic antidepressants compared to newer second-generation antidepressants, use of noradrenergic active antidepressants compared to those that act on serotonin or dopamine, and a history of substance abuse. Read more
Antidepressants Work Better in Major Depressive Disorder than Previously Thought
As we’ve written before, the popular media has sometimes questioned the efficacy of antidepressants for unipolar depression. A reanalysis of data from previous controlled trials of fluoxetine and venlafaxine that was recently published in the Archives of General Psychiatry provides new evidence that these drugs are significantly more efficacious than placebo in youth, adult, and geriatric populations with major depressive disorder.
The researchers concluded,
To our knowledge, this is the first research synthesis in this area to use complete longitudinal person-level data from a large set of published and unpublished studies. The results do not support previous findings that antidepressants show little benefit except for severe depression. The antidepressants fluoxetine and venlafaxine are efficacious for major depressive disorder in all age groups, although more so in youths and adults compared with geriatric patients. Baseline severity was not significantly related to degree of treatment advantage over placebo.
Procedures with Rapid-Onset Antidepressant Effects
 At a recent scientific meeting, Carlos Zarate of the National Institute of Mental Health (NIMH) discussed a variety of rapid-onset antidepressant manipulations. The talk focused on intravenous (IV) administration of the NMDA receptor antagonist ketamine and the anticholinergic drug scopolamine.
At a recent scientific meeting, Carlos Zarate of the National Institute of Mental Health (NIMH) discussed a variety of rapid-onset antidepressant manipulations. The talk focused on intravenous (IV) administration of the NMDA receptor antagonist ketamine and the anticholinergic drug scopolamine.
IV Ketamine
Intravenous ketamine has been administered to approximately 1000 patients at research centers at Mount Sinai Hospital in New York, Yale University, and at the NIMH in Bethesda. The results have been consistent; more than half of the patients experienced a rapid onset of antidepressant effect, usually within the first 2 hours. However, the effects of intravenous ketamine tend to disappear after 3 to 5 days.
In both unipolar and bipolar depressed patients whose illness has been highly resistant to multiple treatments, ketamine also brings about a rapid-onset decrease in suicidal ideation and intent. In some cases these positive effects have lasted a week or more. Thus intravenous ketamine could potentially be used at hospitals to treat suicidal emergencies.
Scopolamine
New data indicate that intravenous scopolamine, a selective blocker of muscarinic cholinergic receptors that is used to help ward off seasickness, brings about onset of antidepressant effects in up to one day and sometimes in a matter of hours. As with ketamine, these effects occur in both unipolar and bipolar depressed patients.
Mechanisms of Action of Ketamine
Editor’s Note: New data from animal studies may be able to explain some of the mechanisms behind the rapid onset of antidepressant effects that occurs with ketamine. Ketamine causes increases in brain-derived neurotrophic factor (BDNF), which is important for the development and health of neurons. In rodents, new synaptic proteins are created following intravenous administration of ketamine.
If animals are subjected to chronic unpredictable stress, dendritic spines (on which synapses are formed) appear to shrink. However, when ketamine is administered intravenously to these animals, not only is their behavior rapidly ameliorated, but the thin dendritic spines shift back to their more mature, mushroomed-shaped form within a few hours. (A slightly slower, less dramatic change in spines occurs with scopolamine.) A loss of dendritic volume has also been demonstrated in depressed people.
The data in animals are not only valuable for their potential application in clinical treatment and emergency situations involving suicidal ideation, but they also demonstrate a mechanism by which a single administration of intravenous ketamine or scopolamine is capable not only of reversing depressive behaviors in animals, but changing dendritic spine morphology. Given this development, it may be possible to identify other treatments that induce rapid-onset antidepressant effects using other parts of the same neurological pathways.
Other Rapid-Onset Approaches: TRH and Sleep Deprivation
Intravenous thyrotropin releasing hormone (TRH) also has rapid-onset antidepressant effects within 24 hours in depressed patients, but tolerance develops after repeated use.
Surprisingly, one night of complete sleep deprivation can also bring about dramatic onset of antidepressant effects the following day in approximately 50% of severely depressed patients.
In animal studies by Ron Duman and colleagues at Yale University, administration of brain-derived neurotrophic factor (BDNF) either into a rodent’s blood or its brain has induced rapid-onset antidepressant-like effects.
Taken together, these data indicate that there are ways to bring about rapid improvement in depression, even if conventional antidepressants usually require 2 to 4 weeks to exert their maximum antidepressant effects. Read more
Mothers’ SSRI Use May Lead to Increased Risk of Pulmonary Hypertension in Infants
The New York Times summarized findings about the effect of mothers’ antidepressant use on enfants from an article published by Kieler et al. in the British Medical Journal this year.
Researchers have long suspected a link between the use of selective serotonin reuptake inhibitors, or S.S.R.I.’s, and [pulmonary hypertension in babies], but previous studies have been small and inconclusive (with results ranging from there being no link to a six times greater risk).
This research, based on 1.6 million births in Denmark, Finland, Iceland, Norway or Sweden from 1996 to 2007, showed that among women using S.S.R.I.’s, the risk of persistent pulmonary hypertension for infants more than doubled (particularly for use late in pregnancy). It’s still a small risk: 3 in 1000 births, as opposed to 1.2 per 1000 births overall. But it’s a small risk of a serious problem.
Pulmonary hypertension, Dr. Juliette Madan, a pediatrician at the Dartmouth Hitchcock Medical Center explained, is diagnosed when an infant struggles to get enough oxygen into her lungs, and therefore into her bloodstream. The condition can be deadly, although Dr. Madan said that it’s usually treatable — with possible lifelong consequences.
But other research suggests that untreated depression during pregnancy has its own risks, including pre-term birth and low birth weight. Given that, how should a pregnant woman and her doctor weigh the competing risks?
See the New York Times for a discussion on how to balance mother’s health with babies’ health.
Tricyclic Antidepressants Linked to Cardiovascular Disease
 Research from 2010 shows that tricyclic antidepressants (TCAs) are linked to 35% greater risk for cardiovascular disease, while selective serotonin reuptake inhibitors (SSRIs) were not shown to confer any extra cardiovascular risks.
Research from 2010 shows that tricyclic antidepressants (TCAs) are linked to 35% greater risk for cardiovascular disease, while selective serotonin reuptake inhibitors (SSRIs) were not shown to confer any extra cardiovascular risks.
Editor’s Note: This is another one of many reasons to use second generation antidepressants such as the SSRIs and bupropion instead of the first generation tricyclics. The TCAs have more side effects, are more dangerous in overdose, are not indicated for children or adolescents, and are more likely to cause switches into mania in individuals with bipolar disorder than the newer ADs.
Common Genetic Variation Linked to Response to Antidepressants
 Brain-derived neurotrophic factor (BDNF) protects neurons and is important for long-term learning and memory. There are several genetic variations in BDNF depending on which amino acid—valine or methionine—falls at a particular position when the proBDNF protein is being made. Most people have the val-66-val allele, some have the val-66-met, and a few have the met-66-met allele.
Brain-derived neurotrophic factor (BDNF) protects neurons and is important for long-term learning and memory. There are several genetic variations in BDNF depending on which amino acid—valine or methionine—falls at a particular position when the proBDNF protein is being made. Most people have the val-66-val allele, some have the val-66-met, and a few have the met-66-met allele.
Researcher Jessica C. Levenson, working with David Kupfer and Ellen Frank at the University of Pittsburgh, reported at the 51st Annual Meeting of the National Institute of Mental Health’s New Clinical Drug Evaluation Unit (NCDEU) in 2011 that patients with unipolar depression who have the val-66-val allele of proBDNF have better clinical responsiveness to antidepressants than those with the slightly less common variant, val-66-met.
Editor’s note: The val-66-val allele is more effective in enhancing synaptic plasticity and is more easily transported from the nucleus to the dendrites of neurons (where it is necessary for learning and memory) than the val-66-met allele or the least effective met-66-met variant.
These findings are intriguing because antidepressant treatments tend to increase BDNF, regardless of their mechanisms of action. Moreover, BDNF levels are low in patients with depression, usually in direct relationship to the severity of depression. Thus, the ability of antidepressants to increase BDNF may lead to a more effective treatment response in those with the better functioning val-66-val allele of BDNF. This remains to be further documented, but the study provides a preliminary example of how genotyping may eventually be able to help predict individual clinical response to a given treatment and thus foster the development of personalized medicine.
Neurological Biomarkers May Eventually Predict Response to Antidepressants
 T.L. Lauriat reported at the 51st Annual Meeting of the National Institute of Mental Health’s New Clinical Drug Evaluation Unit (NCDEU) in 2011 that low baseline levels of the neurotransmitter GABA in the brains of depressed patients were associated with greater response to antidepressants. GABA was measured using magnetic resonance spectroscopy (MRS).
T.L. Lauriat reported at the 51st Annual Meeting of the National Institute of Mental Health’s New Clinical Drug Evaluation Unit (NCDEU) in 2011 that low baseline levels of the neurotransmitter GABA in the brains of depressed patients were associated with greater response to antidepressants. GABA was measured using magnetic resonance spectroscopy (MRS).
These data raise the possibility that easily observed neurobiological markers, such as levels of GABA or the neurotransmitter glutamate, may ultimately be helpful in predicting clinical response to particular treatments.
Clinical Evidence May Explain the Mechanisms of Ketamine’s Rapid Acting Antidepressant Effects
At the 51st Annual Meeting of the National Institute of Mental Health’s New Clinical Drug Evaluation Unit (NCDEU) in 2011, C.G. Abdallah from SUNY Downstate Medical Center reported on a study of intravenous ketamine for treatment-resistant depression. Twelve medication-free participants aged 18-65 received 0.5mg/kg ketamine over 40 minutes. There was a rapid-onset antidepressant effect, as there has been in other studies of unipolar and bipolar depressed patients. In a subgroup of 4 patients examined with magnetic resonance spectroscopy (MRS), there were rapid increases in brain GABA followed shortly thereafter by increases in brain glutamate concentrations.
Editor’s note: The rapid increases in GABA and glutamate that occur after the administration of intravenous ketamine may help account for its therapeutic effects. Other studies have shown that brain GABA is low in depressed patients, so the rapid increase in GABA with ketamine administration could partly explain the antidepressant effects of the drug. The role of the glutamate increases remains to be further explored.
Neli and associates from Yale had reported that in animals, ketamine was able to rapidly alter synapse structure and function. In an animal model of depression, rodents are exposed to chronic and unpredictable stress and develop depressive-like behavior. The mature, mushroom-shaped spines on their dendrites (the parts of neurons that receive synapses and determine the neuron’s excitability) also lose their shape, becoming straighter and spikier like immature spines. Intravenous ketamine not only improves the animals’ behavior, but also increases the number of mushroom-shaped spines within a matter of hours, rapidly improving synaptic function. This effect of ketamine was dependent on a novel intracellular pathway involving the enzyme mTOR, which if blocked prevented the re-emergence of the mature spines.
In the brains of depressed humans studied at autopsy there is reduced neural volume in the frontal cortex, which could possibly be related to dendritic atrophy and associated changes in spine shape as it appears to be in rodents. The animal data suggest the remarkable possibility that intravenous ketamine’s rapid onset of antidepressant effects could also be associated with rapid improvement in the microanatomy of the brain.
The data on ketamine’s effects in animals and the new clinical data showing that GABA and glutamate increases occurred rapidly in depressed patients administered ketamine provide further insight into the potential mechanisms of ketamine’s effect.
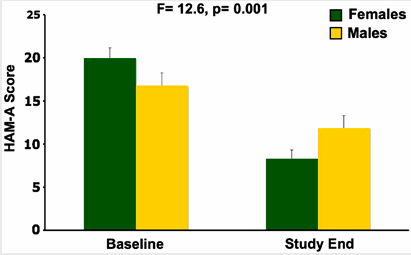
Scopolamine Produces Larger Antidepressant and Antianxiety Effects in Women Than in Men
In a study published in Neuropscyhopharmacology, three sessions of intravenous scopolamine (4µg/kg over 15 minutes) led to rapid antidepressant response in both men and women, but the magnitude of response was larger in women. Women also experienced significant reduction in anxiety, as seen below:
Editor’s Note: Scopolamine is a potent blocker of acetylcholine receptors of the muscarinic type. This can cause side effects such as dry mouth and constipation. However, when given intravenously, scopolamine produces rapid onset of antidepressant effects in both bipolar and unipolar depressed patients. This study suggests that the drug may be even more effective in women.
IV Scopolamine Brings About Rapid Onset Of Antidepressant Effects
 Frankel and colleagues from the National Institute of Mental Health presented a randomized, placebo-controlled clinical trial of intravenous (IV) scopolamine for bipolar depression at the 9th International Conference on Bipolar Disorder (ICBD) in 2011. The same group of investigators previously showed that IV scopolamine was able to induce fast-acting antidepressant responses in those with unipolar major depression. This represents a new and independent study exclusively among patients with bipolar depression.
Frankel and colleagues from the National Institute of Mental Health presented a randomized, placebo-controlled clinical trial of intravenous (IV) scopolamine for bipolar depression at the 9th International Conference on Bipolar Disorder (ICBD) in 2011. The same group of investigators previously showed that IV scopolamine was able to induce fast-acting antidepressant responses in those with unipolar major depression. This represents a new and independent study exclusively among patients with bipolar depression.
In the first phase of the study, three sessions of either IV scopolamine at 4 mcg/kg or a sham treatment were scheduled three to five days apart. In the second phase of the study, the patients were switched to the other treatment for three sessions. The results showed a rapid improvement in depression following the first session with scopolamine, more than occurred with the sham treatment. Hamilton Anxiety Rating scale (HAMA) scores also improved more on scopolamine than with the sham treatment, while Young Mania Rating Scores (YMRS) did not differ.
Editor’s note: As previously discussed in the BNN, scopolamine, an antagonist of acetylcholine muscarinic receptors, appears to exert rapid onset antidepressant effects in both unipolar and bipolar depression. When administered intravenously it takes its place with other rapidly acting antidepressant treatments, including IV ketamine, IV thyrotropin releasing hormone (TRH), and one night of sleep deprivation. The new data indicate that both unipolar and bipolar depression can respond rapidly (i.e. within a matter of hours) to certain treatments, even though most conventionally acting antidepressant modalities can take weeks to achieve maximum antidepressant effects. Read more