Depression and Suicidal Thoughts Linked to Brain Inflammation
A 2017 article by Sophie E. Holmes and colleagues in the journal Biological Psychiatry reports that people with major unipolar depression, especially those with suicidal thoughts, have higher levels of the inflammatory marker translocator protein than do healthy individuals.
The participants with depression and suicidal thinking had high levels of translocator protein in the anterior cingulate cortex, which suggests that inflammation is affecting microglia.
Many studies have found links between different indicators of inflammation and mood disorders, leading researchers to speculate whether targeting the immune system could be an effective way to treat mood disorders. Patients with high levels of inflammation often fail to respond to typical treatments for depression.
Some previous research has found evidence of microglial activation in the brains of people who died from suicide.
The small study by Holmes and colleagues used positron-emission tomography, or PET scans, to observe evidence of translocator protein levels in the brain in 14 medication-free participants in a major depressive episode and 13 healthy volunteers. Those with depression, and particularly those with suicidal thoughts, showed more evidence of neuroinflammation.
Link Clarified Between Gut Microbes and Emotions
A 2017 article in the journal Microbiome suggests that gene-regulating molecules called microRNAs in the brain may be the link between microbes in the gut and emotions.
The research by Alan E. Hoban and colleagues looked at mice raised in a sterile, microbe-free environment. These mice had fewer anxiety-like behaviors than mice raised among the usual bacteria, viruses, and fungi. This finding implies that the microbiome—the trillions of microbes that live in and around our bodies—affects brain functions. In this case, the affected regions were the prefrontal cortex and the amygdala, which both play a role in the detection and response to fearful stimuli. These regions showed alterations in the level of microRNAs present.
When Hoban and colleagues introduced microbes into the animal’s systems, some microRNAs did not bounce back, suggesting that there may be a crucial window early in life when the presence of microbes is needed for the brain to develop normally.
In general, this research shows that microRNAs are key to understanding the link between the microbiome and the brain.
Type of Trauma Affects Gene Transcription Effects in PTSD
 In a 2017 article in the journal Neuropsychopharmacology, researcher Michael S. Breen and colleagues analyzed five separate studies of post-traumatic stress disorder (PTSD) and found that sex and type of trauma affected the immunological pathways that changed with PTSD. People with PTSD showed disruptions in gene expression in specific immunological pathways depending on what type of trauma they had experienced.
In a 2017 article in the journal Neuropsychopharmacology, researcher Michael S. Breen and colleagues analyzed five separate studies of post-traumatic stress disorder (PTSD) and found that sex and type of trauma affected the immunological pathways that changed with PTSD. People with PTSD showed disruptions in gene expression in specific immunological pathways depending on what type of trauma they had experienced.
Men exposed to combat traumas showed downregulation in a pathway related to wound healing, while men who were exposed to interpersonal traumas had upregulation in a signaling pathway mediated by the inflammatory marker IL-12. Women exposed to interpersonal traumas showed upregulation of two pathways—one related to lipid metabolism and the other related to MAPK (or mitogen-activated protein kinase) activity.
The participants with PTSD also showed a lot of the same disruptions across all types of trauma, including disruptions that affected cytokine, innate immune, and type 1 interferon pathways.
These data show that immune dysregulation and inflammatory pathways play a role in the pathophysiology of PTSD.
Different Types of Trauma Affect Brain Volume Differently
Post-traumatic stress disorder (PTSD) has been associated with decreased volume of gray matter in the cortex. Research by Linghui Meng and colleagues has revealed that the specific types of trauma that precede PTSD affect gray matter volume differently.
At the 2016 meeting of the Society for Neuroscience, Meng reported that PTSD from accidents, natural disasters, and combat led to different patterns of gray matter loss. PTSD from accidents was associated with gray matter reductions in the bilateral anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC). PTSD from natural disasters was linked to gray matter reductions in the mPFC and ACC, plus the amygdala and left hippocampus. PTSD from combat reduced gray matter volume in the left striatum, the left insula, and the left middle temporal gyrus.
Meng and colleagues also found that severity of PTSD was linked to the severity of gray matter reductions in the bilateral ACC and the mPFC.
In a 2016 article in the journal Scientific Reports, Meng and colleagues reported that single-incident traumas were associated with gray matter loss in the bilateral mPFC, the ACC, insula, striatum, left hippocampus, and the amygdala, while prolonged or recurrent traumas were linked to gray matter loss in the left insula, striatum, amygdala, and middle temporal gyrus.
Brain Scans Differentiate Suicidal from Non-Suicidal Patients with Bipolar Disorder
 People with bipolar disorder are at high risk for suicidal behavior beginning in adolescence and young adulthood. A 2017 study by Jennifer A. Y. Johnston and colleagues in the American Journal of Psychiatry uses several brain-scanning techniques to identify neurobiological features associated with suicidal behavior in people with bipolar disorder compared to people with bipolar disorder who have never attempted suicide. Clarifying which neural systems are involved in suicidal behavior may allow for better prevention efforts.
People with bipolar disorder are at high risk for suicidal behavior beginning in adolescence and young adulthood. A 2017 study by Jennifer A. Y. Johnston and colleagues in the American Journal of Psychiatry uses several brain-scanning techniques to identify neurobiological features associated with suicidal behavior in people with bipolar disorder compared to people with bipolar disorder who have never attempted suicide. Clarifying which neural systems are involved in suicidal behavior may allow for better prevention efforts.
The study included 26 participants who had attempted suicide and 42 who had not. Johnston and colleagues used structural, diffusion tensor, and functional magnetic resonance imaging (MRI) techniques to identify differences in the brains of attempters and non-attempters.
Compared to those who had never attempted suicide, those who had exhibited reductions in gray matter volume in the orbitofrontal cortex, hippocampus, and cerebellum. They also had reduced white matter integrity in the uncinate fasciculus, ventral frontal, and right cerebellum regions. In addition, attempters had reduced functional connectivity between the amygdala and the left ventral and right rostral prefrontal cortex. Better right rostral prefrontal connectivity was associated with less suicidal ideation, while better connectivity of the left ventral prefrontal area was linked to less lethal suicide attempts.
Breathing in Through the Nose Enhances Judgment and Memory
A 2016 study published in the Journal of Neuroscience reported that the rhythm of breathing changes electrical activity in the brain and can improve emotional judgments and recall. Breathing in through the nose seemed to produce benefits compared to breathing out or to breathing in through the mouth.
Participants more easily identified a fearful face if they viewed it while breathing in. They also had an easier time remembering objects they observed while breathing in. The effects were not seen if the participants breathed through their mouth.
The researchers, led by Christina Zelano, reported that there was a major difference in brain activity in the amygdala and hippocampus during inhalation versus exhalation. Breathing in, in addition to stimulating the olfactory cortex responsible for smell perception, seems to activate the entire limbic system, the emotional center of the brain.
Levels of Amino Acid Proline Interact with COMT Genotype to Affect Negative Symptoms
 In a 2016 article, researcher Catherine L. Clelland and colleagues reported that a patient’s levels of the amino acid proline interact with their genetic profile to influence the seriousness of their negative symptoms. Negative symptoms of schizophrenia and bipolar disorder include flat affect and lack of volition and can be some of the hardest symptoms to treat.
In a 2016 article, researcher Catherine L. Clelland and colleagues reported that a patient’s levels of the amino acid proline interact with their genetic profile to influence the seriousness of their negative symptoms. Negative symptoms of schizophrenia and bipolar disorder include flat affect and lack of volition and can be some of the hardest symptoms to treat.
High levels of proline in the central nervous system have been linked to schizophrenia. Proline is a precursor to the neurotransmitter glutamate, and high proline levels have been found to alter glutamate and dopamine signaling in mice. This is one of the factors affecting negative symptoms.
The other factor affecting negative symptoms is the COMT gene. The enzyme catechol-o-methlyl transferase (COMT) metabolizes dopamine in the prefrontal cortex. There are several common versions of the gene for COMT. The most efficient is known as val-158-val, identifying that the gene has two valine amino acids at position 158. People with high proline levels and the val-158-val version of the COMT gene had fewer negative symptoms than people with high proline levels and another version of the gene, val-158-met (indicating one valine and one methionine amino acid at position 158).
Clelland and colleagues hypothesized that high proline levels may actually counteract the dopamine shortages common in the prefrontal cortex in people with the val-158-val genotype of COMT, which is more efficient at breaking down dopamine in this region.
The mood stabilizer valproate increases proline levels. In the study, which was published in Translational Psychiatry, people with schizophrenia and the val-val genotype had fewer negative symptoms when treated with valproate than those with the val-met genotype who received the same treatment.
Specific Regions of Hippocampus Linked to Bipolar Disorder
It has been clear for some time that the volume of the hippocampus, a brain region implicated in mood and memory processing, plays a role in bipolar disorder. A 2017 article by researcher Bo Cao and colleagues in the journal Molecular Psychiatry links loss of volume in specific sub-regions of the hippocampus with bipolar disorder.
The study by Cao and colleagues used magnetic resonance imaging (MRI) and a special segmentation technique to compare the volume of certain hippocampal sub-regions across people with bipolar disorder, people with major depression, and healthy control participants.
Participants with bipolar disorder had lower volumes in subfield 4 of the cornu ammonis, two cellular layers (the granule cell layer and the molecular layer), and the tail part of the seahorse-shaped hippocampus compared to the other subjects. Participants with bipolar I disorder had particularly severe volume loss in these areas.
Cao and colleagues also found that volume loss progressed along with the illness. The volumes of the right cornu ammonis, the molecular layer, and the subiculum decreased further in patients who had bipolar disorder for longer. As manic episodes increased, the volume of both sides of the cornu ammonis and the hippocampal tail decreased.
Lithium May Work by Restoring Dendritic Spines
New research on mice clarifies lithium’s effects on neurons and suggests how it can lead to improved symptoms. Dendrites are the long projections on neurons that seem to reach out to form synapses with other neurons. The surface of these dendrites is covered in mushroom-shaped spines that help create these synaptic connections. A 2016 article by research Ben Cheyette and colleagues in the journal Molecular Psychiatry reports that in mice with a genetic mutation common to people with autism, schizophrenia, and bipolar disorder, lithium restored healthy numbers of the mushroom-shaped spines. The lithium treatment also reversed symptoms such as lack of interest in social interactions, lack of motivation, and anxiety in the mice.
Cheyette and colleagues first identified a genetic mutation that affects signaling in what is known as the brain’s Wnt pathway. The mutation, while rare, is 80% more common in people with bipolar disorder, autism, and schizophrenia than in people without these disorders.
When the mice were given a similar mutation, they exhibited symptoms such as anxiety, decreased sociability, and lack of motivation. They also had reduced numbers of dendritic spines and impaired Wnt signaling.
Lithium can improve Wnt signaling by blocking an enzyme called GSK-3 beta that impairs the signaling.
Treating the mice with lithium restored their dendritic spines and improved their behavior.
Wnt signaling and dendritic spines may offer the key to lithium’s success in treating a variety of psychiatric disorders in people.
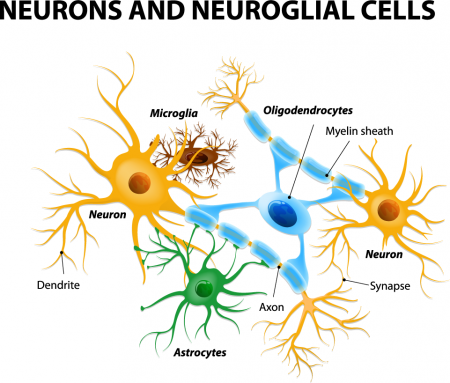
Astrocytes Can Turn Toxic
Astrocytes usually play a helpful role in the brain. These glial cells facilitate neural connections and prune unnecessary ones. However, new research details how infection or trauma can render astrocytes toxic, leading to brain disorders. In a 2017 article in the journal Nature, researcher Shane A. Liddelow and colleagues describe how resting astrocytes can become harmful reactive astrocytes.
The researchers determined that reactive astrocytes are found in brain tissues following brain injuries, or in neurological disorders including Alzheimer’s, Parkinson’s, Huntington’s, amyotrophic lateral sclerosis (ALS), and multiple sclerosis. Liddelow and colleagues also determined that microglia play a role in transforming resting astrocytes into a subtype of harmful reactive astrocytes, dubbed A1 astrocytes, and that the latter secrete a neuron-killing toxin.
A1 astrocytes lose their ability to promote neuronal survival and to create new synapses. The researchers showed that blocking the formation of A1 astrocytes prevented certain neuron deaths, and they hope this finding will lead to new treatments for brain injuries and neurological disorders.
Editor’s Note: It is possible that A1 astrocytes are also induced in patients with bipolar disorder, post-traumatic stress disorder (PTSD), and schizophrenia, as well as head traumas and neurological disorders.