Specific Regions of Hippocampus Linked to Bipolar Disorder
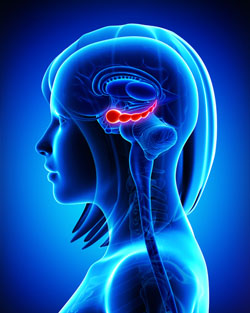
It has been clear for some time that the volume of the hippocampus, a brain region implicated in mood and memory processing, plays a role in bipolar disorder. A 2017 article by researcher Bo Cao and colleagues in the journal Molecular Psychiatry links loss of volume in specific sub-regions of the hippocampus with bipolar disorder.
The study by Cao and colleagues used magnetic resonance imaging (MRI) and a special segmentation technique to compare the volume of certain hippocampal sub-regions across people with bipolar disorder, people with major depression, and healthy control participants.
Participants with bipolar disorder had lower volumes in subfield 4 of the cornu ammonis, two cellular layers (the granule cell layer and the molecular layer), and the tail part of the seahorse-shaped hippocampus compared to the other subjects. Participants with bipolar I disorder had particularly severe volume loss in these areas.
Cao and colleagues also found that volume loss progressed along with the illness. The volumes of the right cornu ammonis, the molecular layer, and the subiculum decreased further in patients who had bipolar disorder for longer. As manic episodes increased, the volume of both sides of the cornu ammonis and the hippocampal tail decreased.
Lithium May Work by Restoring Dendritic Spines
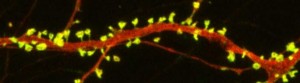
New research on mice clarifies lithium’s effects on neurons and suggests how it can lead to improved symptoms. Dendrites are the long projections on neurons that seem to reach out to form synapses with other neurons. The surface of these dendrites is covered in mushroom-shaped spines that help create these synaptic connections. A 2016 article by research Ben Cheyette and colleagues in the journal Molecular Psychiatry reports that in mice with a genetic mutation common to people with autism, schizophrenia, and bipolar disorder, lithium restored healthy numbers of the mushroom-shaped spines. The lithium treatment also reversed symptoms such as lack of interest in social interactions, lack of motivation, and anxiety in the mice.
Cheyette and colleagues first identified a genetic mutation that affects signaling in what is known as the brain’s Wnt pathway. The mutation, while rare, is 80% more common in people with bipolar disorder, autism, and schizophrenia than in people without these disorders.
When the mice were given a similar mutation, they exhibited symptoms such as anxiety, decreased sociability, and lack of motivation. They also had reduced numbers of dendritic spines and impaired Wnt signaling.
Lithium can improve Wnt signaling by blocking an enzyme called GSK-3 beta that impairs the signaling.
Treating the mice with lithium restored their dendritic spines and improved their behavior.
Wnt signaling and dendritic spines may offer the key to lithium’s success in treating a variety of psychiatric disorders in people.
Astrocytes Can Turn Toxic
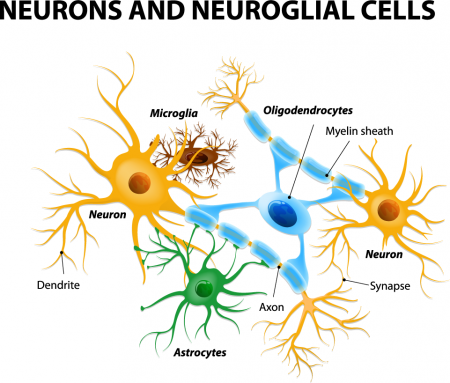
Astrocytes usually play a helpful role in the brain. These glial cells facilitate neural connections and prune unnecessary ones. However, new research details how infection or trauma can render astrocytes toxic, leading to brain disorders. In a 2017 article in the journal Nature, researcher Shane A. Liddelow and colleagues describe how resting astrocytes can become harmful reactive astrocytes.
The researchers determined that reactive astrocytes are found in brain tissues following brain injuries, or in neurological disorders including Alzheimer’s, Parkinson’s, Huntington’s, amyotrophic lateral sclerosis (ALS), and multiple sclerosis. Liddelow and colleagues also determined that microglia play a role in transforming resting astrocytes into a subtype of harmful reactive astrocytes, dubbed A1 astrocytes, and that the latter secrete a neuron-killing toxin.
A1 astrocytes lose their ability to promote neuronal survival and to create new synapses. The researchers showed that blocking the formation of A1 astrocytes prevented certain neuron deaths, and they hope this finding will lead to new treatments for brain injuries and neurological disorders.
Editor’s Note: It is possible that A1 astrocytes are also induced in patients with bipolar disorder, post-traumatic stress disorder (PTSD), and schizophrenia, as well as head traumas and neurological disorders.
How Stress Triggers Inflammation and Depression
 Depression and bipolar disorder are associated with increases in markers of inflammation that can be found in the brain and blood. It is increasingly clear that the mechanisms that cause depression are not just in the brain, but actually throughout the body. These include two signaling systems that begin in the bone marrow and the spleen.
Depression and bipolar disorder are associated with increases in markers of inflammation that can be found in the brain and blood. It is increasingly clear that the mechanisms that cause depression are not just in the brain, but actually throughout the body. These include two signaling systems that begin in the bone marrow and the spleen.
When a small mouse is repeated defeated by a larger animal, they show depression-like symptoms known as defeat stress. Animal studies have shown that stress and danger signals are perceived and relayed to the amygdala and the hypothalamus. The sympathetic nervous system releases the neurotransmitter norepinephrine into bone marrow, where stem cells are turned into activated monocytes (a type of white blood cells) that are then released into the blood. The monocytes travel to the brain, leading to the activation of more inflammatory cells.
Blocking part of this process can prevent the depression-like behaviors from occurring. If the bone marrow monocytes are blocked from entering the brain, inflammation and defeat stress behaviors like social avoidance do not occur. However, if there is a second bout of defeat stress, primed monocytes that have been stored in the spleen are released and travel to the brain, producing further increases in inflammatory cells and even more defeat stress behaviors.
If these monocytes from the spleen are blocked, the inflammation and the reaction to the new stressor do not occur.
Stress also activates lymphocytes (another type of white blood cells) to secrete the inflammatory cells Il-6. If this Il-6 secretion is inhibited, defeat stress behaviors can be prevented.
Defeat stress also leads to the release of the neurotransmitter glutamate. Some of this cascade begins in the brain, which evaluates stressors and releases IL-1 beta, another type of inflammatory cell. It slows down the production of glutamate, while IL-6 can endanger neurons and is associated with anhedonia—loss of interest in pleasurable activities. This cascade also leads to the production of another type of inflammatory cell, TNF-alpha, which has adverse effects on biochemistry, brain, and behavior.
This understanding of the role of the brain and body provides new targets for drug development. If inflammatory processes are blocked, defeat stress behaviors do not occur. Researcher Michael D. Weber and colleagues described this process in detail in the journal Neuropsychopharmacology Reviews in 2017.
Together these observations suggest that inflammatory processes in the body are crucial to the development of some stress- and inflammation-related depressive behaviors.
Inflammatory Marker CRP Higher in Bipolar Disorder, Particularly Mania
Inflammation has been linked to mood disorders. A 2016 meta-analysis in the journal Lancet Psychiatry described the role of inflammatory marker C-reactive protein (CRP) in bipolar episodes. Researchers led by Brisa S. Fernandes identified 27 studies that measured CRP levels in a total of 2,161 patients with bipolar disorder and 81,932 healthy participants. The researchers determined that compared to healthy controls, people with bipolar disorder have higher levels of CRP. CRP levels were moderately elevated between episodes and during depression, and substantially elevated during episodes of mania.
Editor’s Note: This meta-analysis shows that CRP is linked to bipolar disorder, and the inflammatory burden is highest during mania. It remains to be seen whether anti-inflammatory treatments work best in patients with high CRP levels compared to normal CRP levels.
CRP is also a risk factor for cardiovascular disease, and lithium and other treatments for bipolar disorder probably lower CRP levels.
The same group of researchers previously showed that statins, drugs typically used to lower cholesterol, could help alleviate depression. Since statins have anti-inflammatory effects, they can probably reduce depression risk in addition to lowering cardiovascular risk, as initial studies suggest.
Other drugs with anti-inflammatory effects that may improve depression include the anti-arthritis drug celecoxib and the antibiotic minocycline. The amino acid N-acetylcysteine and omega-3 fatty acids also have anti-inflammatory effects and have been found to improve depression in some studies.
Kynurenine Pathway Suggests How Inflammation is Linked to Schizophrenia
The kynurenine pathway describes the steps that turn the amino acid tryptophan (the ingredient in turkey that might make you sleepy) into nicotinamide adenine dinucleotide. This pathway might be a connection between the immune system and neurotransmitters involved in schizophrenia.
A recent autopsy study by researcher Thomas Weickert and colleagues explored this link by determining that in the brains of people with schizophrenia and high levels of inflammation, messenger RNA for Kynurenine Aminotransferase II (KATII, a step on the kynurenine pathway) was elevated in the dorsolateral prefrontal cortex compared to the brains of people who died healthy and those with schizophrenia but low levels of inflammation.
The KATII mRNA levels also correlated with mRNA levels of inflammatory markers such as glial fibrillary acidic protein and interleukin-6.
Blood measures related to the kynurenine pathway also differentiated people with schizophrenia from healthy controls. People with schizophrenia had lower levels of tryptophan, kynurenine, and kynurenic acid in their blood. The low levels of kynurenic acid in the blood were correlated with deficits in working memory and smaller volume of the dorsolateral prefrontal cortex.
Weickert and colleagues suggest that blood levels of kynurenic acid might provide a measurable indicator of the degree to which people with schizophrenia are experiencing problems with executive functioning (planning and decision-making) and loss of brain volume.
Brain Inflammation Found in Autopsy Studies of Teen and Adult Suicides
Suicide and depression have both been linked to elevated levels of inflammatory cytokines in the blood and cerebrospinal fluid. A recent study finds that these inflammatory markers are also elevated in the brains of teens and depressed adults who died from suicide.
In autopsy studies, researcher Ghanshyam N. Pandey measured levels of the inflammatory cytokines interleukin-1beta, interleukin 6, and TNF-alpha in the brains of teen suicide victims, and compared these to the brains of teens who died from other causes. Pandey also measured levels of interleukin-1beta, interleukin 6, interleukin 8, interleukin 10, interleukin 13, and TNF-alpha in the prefrontal cortex of depressed adult suicide victims and compared them to levels in adults who died of other causes.
There were abnormalities in the inflammatory markers in the brains of those who died from suicide compared to their matched controls. The suicide victims had higher levels of interleukin-1beta, interleukin 6, and TNF-alpha than the controls. Among the adults, levels of the anti-inflammatory cytokine interleukin 10 were low in the suicide victims while levels of Toll-like receptors (TLR3 and TLR4), which are involved in immune mechanisms, were high.
Brain inflammation has also been observed in positron emission tomography (PET) scans of depressed patients, where signs of microglial activation can be observed. Elevated inflammatory cytokines are also found in the blood of some people with bipolar disorder, depression, and schizophrenia.
Pandey presented this research at the 2016 meeting of the Society of Biological Psychiatry.
In Rats, Dad’s Cocaine Use Affects Son’s Spatial Memory
Evidence is mounting that certain behaviors by parents can leave marks on their sperm or eggs that are passed on to their offspring in a process called epigenetics. In a recent study by researcher Mathieu Wimmer and colleagues, male rats that were exposed to cocaine for 60 days (the time it takes for sperm to develop fully) had male offspring who showed diminished short- and long-term spatial memory compared to the offspring of male rats that were not exposed to cocaine. Female offspring were not affected in this way.
The spatial tasks the offspring rats completed depended heavily on the hippocampus. Wimmer and colleagues believe that cocaine use in the fathers decreased the amount of a brain chemical called d-serine in the offspring. D-serine plays a role in memory formation and the brain’s ability to form synaptic connections. Injecting the offspring of rats who were exposed to cocaine with d-serine before the spatial memory tasks normalized the rats’ performance.
Cognitive Behavioral Therapy Improves Depression, PTSD by Improving Brain Connectivity
A recent study clarified how cognitive behavioral therapy improves symptoms of depression and post-traumatic stress disorder (PTSD). The participants were 62 adult women. One group had depression, one had PTSD, and the third was made up of healthy volunteers. None were taking medication at the time of the study. The researchers, led by Yvette Shelive, used functional magnetic resonance imaging (fMRI) to analyze participants’ amygdala connectivity.
At the start of the study, participants with depression or PTSD showed diminished connectivity between the amygdala and brain areas related to cognitive control, the process by which the brain can vary behavior and how it processes information in the moment based on current goals. The lack of connectivity reflected the severity of the participants’ depression. Twelve weeks of cognitive behavioral therapy improved mood and connectivity between the amygdala and these control regions, including the dorsolateral prefrontal cortex and the inferior frontal cortex. These regions also allow for executive functioning, which includes planning, implementation, and focus.
Inflammation Plays a Role in Fear Memories
People with post-traumatic stress disorder (PTSD) often experience fearful memories of the trauma they witnessed. Researchers are working to determine the neurobiological basis for these persistent fear memories in order to better treat PTSD. Current treatments mainly target the central nervous system. Because many people with PTSD have elevated levels of pro-inflammatory immune molecules in their blood, there has been a recent push to determine whether targeting that inflammation may be another way of treating PTSD.
A recent study by researchers Matthew Young and Leonard Howell used an animal model to learn more about the link between trauma, inflammation, and fear memories. The researchers exposed mice to a trauma that produced both a persistent fear response and an increase in inflammatory molecules in the blood. Some of the mice were also given antibodies to neutralize the inflammatory immune response. When the mice were exposed to a cue meant to remind them of the trauma, levels of the inflammatory molecule IL-6 spiked again. When the mice were given antibodies to neutralize IL-6 just before being exposed to the cue, they produced less of a fear reaction.
The researchers, who presented their work at a scientific meeting in December, concluded that traumatic experiences produce not only persistent fearful memories, but also an immune reaction. They believe that the spike in IL-6 following trauma plays a role in the persistence of those memories, and that elevated IL-6 in the blood may explain symptoms of PTSD and other disorders that involve fear learning (such as phobias).