Psilocybin Comparable to Escitalopram in the Treatment of Depression

Four randomized, controlled clinical trials have established that psilocybin, the hallucinogenic compound in “magic mushrooms,” has anti-depressant effects. Recently, a phase 2 clinical trial compared the effects of psilocybin to a US Food and Drug Administration (FDA)–approved treatment for depression, the selective serotonin-reuptake inhibitor (SSRI) escitalopram. The study by Robin Carhart-Harris and colleagues was published in the New England Journal of Medicine in 2021.
The 59 participants in the 6-week study, which took place in the United Kingdom, had longstanding moderate-to-severe major depressive disorder. They were randomized to two groups. The psilocybin group received two separate doses of 25 mg of psilocybin 3 weeks apart, plus 6 weeks of daily placebo. The escitalopram group received two separate doses of 1 mg of psilocybin 3 weeks apart plus 6 weeks of daily oral escitalopram. (The small doses of psilocybin administered to the escitalopram group were assumed to have negligible psychiatric effects.) All participants were told they would receive psilocybin (though not the dose) in order to standardize their expectations.
In addition to the drug treatments, all participants also received psychological support, which consisted of monitoring immediately after the administration of the drugs (given the expectation that the 25mg dose of psilocybin would produce an “altered quality of conscious experience”), psychological debriefings, and an active listening session.
Psilocybin improved depression symptoms to a greater extent than escitalopram did. Among the participants, 70% of the psilocybin group responded to the treatment, compared with 48% of the escitalopram group. Remission occurred in 57% of the psilocybin group compared with 28% of the escitalopram group. These differences in the outcomes for the two groups were not statistically significant.
The FDA has designated psilocybin a “Breakthrough Therapy” for severe depression, which indicates that the therapy may be a substantial improvement over existing therapies for a serious condition. The designation is intended to speed up the drug development and review process.
The state of Oregon legalized psilocybin-assisted therapy in 2020.
MDMA Superior to Placebo in Treatment of Severe PTSD

An article by researcher Jennifer M. Mitchell and colleagues published in the journal Nature in 2021 reported that MDMA was more effective than placebo at treating severe post-traumatic stress disorder in an 18-week phase 3 clinical trial.
The article reported on a randomized, double-blind, placebo-controlled, multi-site trial that took place in the US, Canada, and Israel. Participants in the trial had severe PTSD, and in some cases also had dissociation, depression, a history of alcohol or substance use disorders, or childhood trauma. In the trial, 91 participants were required to stop any current psychiatric medications for a “washout” period, and then were randomized to either a group that received 12 therapy sessions plus placebo or a group that received 12 therapy sessions and 3 doses of MDMA, which were administered in a clinical setting.
Two months after the last therapy session, participants who had received MDMA during the trial showed greater reductions in PTSD symptoms and less functional impairment than those who were assigned to the placebo group during the trial. At the endpoint of the study, 67% of participants in the MDMA group no longer met the criteria for a PTSD diagnosis, compared to 32% of the placebo group.
Side effects that were more prevalent among the MDMA group were typically transient and mild to moderate in severity. These included muscle tightness, decreased appetite, nausea, sweating, feeling cold, and increased blood pressure and heartrate. The MDMA group did not show any increases in drug abuse or suicidality compared with the placebo group, and MDMA had similar effects among those participants with and without comorbid conditions. The authors concluded that MDMA was highly efficacious, safe, and well-tolerated as a treatment for PTSD and urged that further study of the treatment be done without delay.
Currently, US Food and Drug Administration (FDA)–approved treatments for PTSD include the selective serotonin reuptake inhibitors (SSRIs) sertraline and paroxetine, but approximately half of PTSD patients do not respond to these drugs or to evidence-based trauma-specific psychotherapy or cognitive-behavioral therapies. The FDA has designated MDMA-assisted therapy for PTSD a “Breakthrough Therapy.” This designation indicates that the therapy may be a substantial improvement over existing therapies for a serious condition, and is intended to speed up the drug development and review process.
MDMA (or 3,4-methylenedioxymethamphetamine) is the same chemical used recreationally under the name ecstasy or molly. However, the positive effects in the trial followed careful dosing (which is impossible with street drugs) and targeted therapy sessions.
Transcranial Direct Current Stimulation (tDCS) Induces Gray-Matter Increases in Depression
At the 2021 meeting of the Society of Biological Psychiatry (SOBP), researcher Mayank Jog and colleagues described a study of transcranial direct current stimulation (tDCS) in 59 patients with moderate depression. The patients received either tDCS sessions that delivered electrical current at 2mA for 20 minutes or a same-length sham stimulation delivered using a double-blind stimulator, for a total of 12 sessions over 12 consecutive working days. Jog and colleagues found that compared to the sham stimulation, tDCS induced increases in gray matter volume in the left dorsolateral prefrontal cortex (DLPFC) target area, with a statistically large effect size (Cohen’s d = 1.3). The researchers plan to follow up this study that found structural changes to the brain after tDCS with more research on the antidepressant effects of the treatment.
Good Outcomes Among Patients with Major Depressive Disorder Treated with Transcranial Magnetic Stimulation in a Large Study
At the 2021 meeting of the Society of Biological Psychiatry (SOBP), researcher Harold Sackeim and colleagues reported on data collected from patients in clinical treatment for major depression who received transcranial magnetic stimulation (TMS) at 103 practice sites. A total of 5,010 depressed patients were included in the intent-to-treat sample, and 3,814 completed the study, meaning that they either reached remission or were treated at least 20 times and went through a final assessment. “Response (58–83%) and remission (28–62%) rates were notably high across self-report and clinician-administered assessments,” and women had better outcomes than men. Sackeim and colleagues concluded, “Strong efficacy and the low side effect and medical risk profile suggest that TMS be evaluated as a first-line treatment for [major depressive disorder].”
Accelerated Intermittent Theta-Burst Stimulation (aiTBS) Quickly Improved Treatment-Resistant Depression
At the 2021 meeting of the Society of Biological Psychiatry (SOBP), researcher Nolan Williams and colleagues described a sham-controlled trial of accelerated intermittent theta-burst stimulation (aiTBS) in patients with treatment-resistant depression. Theta burst stimulation is a specific protocol for rTMS, or repeated transcranial magnetic stimulation, a non-invasive form of brain stimulation. Magnets placed on a patient’s head provide bursts of high-frequency stimulation to the brain.
Participants in the study received 50 sessions over 5 days of either aiTBS or a sham procedure. Among those who received the real aiTBS treatment, 85.7% saw an improvement in their treatment-resistant depression, compared to only 26.7% of those in the sham group. AiTBS produced a rapid antidepressant response, which Williams and colleagues suggest could be useful for the treatment of patients in emergency rooms or inpatient settings.
Transcranial Near-Infrared Light May Treat Brain Injury and Neurodegeneration
At the 2021 meeting of the Society of Biological Psychiatry, there was a symposium on treatment with near-infrared light chaired by researchers Paolo Cassano and Dan Iosifescu. The treatment is known as transcranial photobiomodulation (PBM) with near-infrared light. A device worn on the head delivers infrared light that penetrates the cerebral cortex and can modulate cortical excitability. It has a variety of effects including promoting neuroplasticity, improving oxygenation, and decreasing inflammation and oxidative stress.
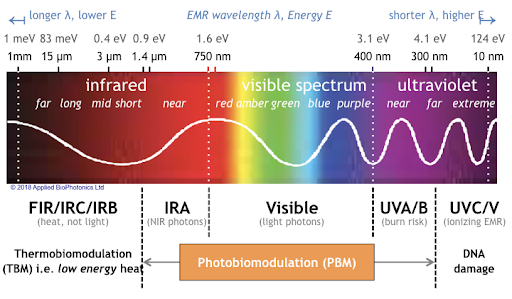
A number of studies exploring the possibility that PBM could be used as a clinical treatment for conditions such as depression, brain injury, or dementia were presented at the symposium.
Researcher Lorelei Tucker discussed promising findings from an animal model in which rats with stroke or brain injuries showed improvement after being treated with PBM.
Cassano discussed studies aimed at refining which brain areas should be targeted with PBM and how much light should be delivered in order to improve depression. In double-blind, sham-controlled studies in people with major depression, targeting the dorsolateral prefrontal cortex with PBM improved their symptoms.
Researcher Benjamin Vakoc discussed a study of low-level light therapy (LLLT) using near-infrared light compared to a sham procedure in 68 people with moderate traumatic brain injury. The researchers used magnetic resonance imaging (MRI) to assess changes in white matter in the brain over time in people recovering from an acute brain injury. Patterns of changes in white matter were different for those who received LLLT compared to those who received the sham procedure.
Researcher Linda Chao described a very small study to determine whether PBM could improve symptoms of dementia. Four patients received typical dementia care while four others underwent home treatments with the commercially available Vielight Neuro Gamma device, which delivers PBM via both the scalp and an insert in the nose. After 12 weeks, the PBM group showed improvements in cognition and brain connectivity.

Editor’s Note: We will be watching the literature to follow advances in this promising novel method of neuromodulation.
Pilot Study of Pramipexole for Anhedonia Symptoms in Unipolar Depression

At a recent scientific meeting, researcher Laura Hack described an open-label pilot study of pramipexole in participants with major depression including anhedonia, or inability to feel pleasure. Five participants with prominent anhedonia and major depression completed eight weeks of treatment with pramipexole, which activates dopamine D2 and D3 receptors. Four of the five who completed the study saw notable improvements in their anhedonia and quality of life.
The starting daily dose was 0.26 mg, which was increased every three days to a target dose of 2.0 mg per day. Two additional patients dropped out due to side effects (nausea, poor sleep, and headache).
Low activity in the ventral striatum, a part of the brain associated with decision-making and implicated in the brain’s reward system, correlated with the severity of the patients’ anhedonia. These results suggest that further studies are warranted.
Pramipexole has also shown positive effects in two small studies in bipolar depression. The drug is currently approved by the US Food and Drug Administration for the treatment of Parkinson’s disease and also works in restless legs syndrome.
A Novel Drug Shows Promise in the Treatment of Negative Symptoms of Schizophrenia

At a recent scientific meeting, Kenneth Koblan, Chief Scientific Officer at Sunovion Pharmaceuticals, Inc. reported on a new drug in development, SEP-363856, and its effects on negative symptoms of schizophrenia.
In both a 4-week double-blind study of participants with acute schizophrenia and in an open (non-blind) 6-month extension study, SEP-363856 was effective on negative symptoms. In the placebo-controlled acute study, those randomized to 50mg or 75mg of the drug showed improvement of moderate effect size in the following symptoms: blunted affect, avolition, anhedonia, asociality, and alogia. In the extension study, which consisted of 26 weeks of treatment with flexible doses (25/50/75 mg/day) of SEP-365846, patients improved further on multiple scales measuring negative symptoms.
SEP-363856 is a novel trace amine receptor 1 (TAAR1) agonist with serotonin 5-HT1A activity. Koblan and colleagues concluded, “These results suggest that activation of the TAAR1 receptor by SEP-363856, in the absence of D2 receptor blockade, may represent a promising approach to the treatment of negative symptoms in schizophrenia.”
Pimavanserin Prevents Relapse in Patients with Dementia-Related Psychosis

At a recent scientific meeting, Erin Foff of Acadia Pharmaceuticals Inc. described a study of pimavanserin (a selective serotonin inverse agonist/antagonist at 5-HT2A receptors) in dementia-related psychosis. Pimavanserin is currently approved in the United States for the treatment of hallucinations and delusions associated with Parkinson’s disease (PD). There is currently no Food and Drug Administration–approved treatment for dementia-related psychosis.
Enrolled patients had moderate-to-severe psychosis associated with Alzheimer’s disease, Parkinson’s, dementia with Lewy bodies, vascular dementia, or frontotemporal dementia. After a 12-week open label phase with flexible dosing and a target dosage of 34mg/day, 217 of the participants with a good response to pimavanserin were then randomized to continue pimavanserin or switch to placebo. The study was stopped early when a prespecified interim analysis revealed that pimavanserin was clearly superior to placebo. There was a more than 2.8-fold reduction in risk of relapse with pimavanserin compared to placebo in the double-blind period. Those on higher doses of 34mg/day showed a more than 3.4-fold reduced risk of relapse. Acadia will seek FDA approval for pimavanserin for the treatment of dementia-related psychosis.
Lumateperone Improves Bipolar Depression Symptoms

At a recent scientific meeting, Suresh Durgam of Intra-Cellular Therapies, Inc. reported on a study of lumateperone tosylate for the treatment of bipolar depression. Lumateperone tosylate is a mechanistically novel antipsychotic that has been approved by the US Food and Drug Administration for the treatment of schizophrenia.
In a double-blind, placebo-controlled study, the drug showed efficacy in bipolar I and II depression. In a 6-week study, 377 patients received either 42 mg/day of lumateperone or placebo, and 333 (87.4%) completed treatment. Lumateperone treatment significantly improved total scores on the Montgomery Asberg Depression Rating Scale (MADRS) compared with placebo. Item analysis revealed that 8 of 10 MADRS items improved significantly in comparison with placebo by day 29, and all items did by day 43. The largest effects were in reported sadness, apparent sadness, inner tension and reduced sleep. Durgam and colleagues concluded that lumateperone at a dose of 42mg improves a broad range of symptoms in bipolar I and bipolar II depression.

